Translate this page into:
A comprehensive review of pathogenesis of mucormycosis with implications of COVID-19: Indian perspective

*Corresponding author: Sanpreet Singh Sachdev, Department of Oral Pathology and Microbiology, Government Dental College and Hospital, Mumbai, Maharashtra, India. sunpreetss@yahoo.in
-
Received: ,
Accepted: ,
How to cite this article: Sachdev SS, Chettiankandy TJ, Sardar MA, Ramaswamy E, Shah AM, Yaduwanshi K. A comprehensive review of pathogenesis of mucormycosis with implications of COVID-19: Indian perspective. J Global Oral Health 2021;4:116-22.
Abstract
Mucormycosis is a deadly fungal infection that targets immunocompromised individuals. India being the “diabetes” capital of the world contributes to about 40% of global burden of the infectious disease. With the addition of COVID-19 pandemic to the equation, there has been an alarming increase in the number of reported cases of mucormycosis. The disease itself along with corticosteroid and certain other strategies used in its treatment predispose the patients to develop secondary bacterial and fungal infections. Therefore, it is imperative for clinicians to understand the pathogenesis of mucormycosis at present so that they can develop combative counter strategies. We provide a comprehensive review of the pathogenetic process of mucormycosis while also elucidating implications of COVID-19 pandemic in the epidemiology and pathogenesis of the infectious malice in an Indian background.
Keywords
Mycoses
Osteomyelitis
Diabetes mellitus
Glucocorticoids
Rhizopus
INTRODUCTION
Ever since its inception, the COVID-19 pandemic has incessantly involved populations from all the countries with more than 156 million cases globally. With more than 21 million cases and 2 million confirmed deaths associated with COVID-19, India has suffered the cataclysmic effects of the pandemic with number of cases continually rising even at present.[1] The disease has an immense physical, financial, and psychological impact on the population. At present, glucocorticoids and remdesivir are the most commonly employed drugs to reduce the mortality in patients with COVID-19 having hypoxemia.[2] Nevertheless, steroid therapy leads to a definite compromise in the patient’s immune capacity which is further aggravated by the impaired cell-mediated immune response and multiorgan dysfunction caused by the disease itself.[3]
The immunocompromised state generated opens way for various secondary bacterial and fungal opportunistic infections. Invasive fungal infections account for a significant portion of morbidity and mortality in immunocompromised patients in which Candida spp., Aspergillus spp., Zygomycetes, and Cryptococcus neoformans have been identified as the most common etiological agents.[4] The main “perfect fungi” causing oral infections consist of Aspergillus, Geotrichium, and Mucor spp. An alarming rise in cases of mucormycosis that is caused by fungi of the order Mucorales has been reported globally which is a deadly infectious disease.[5]
Although infection from Mucorales is resisted and dismissed by normal immunological responses in an immunocompetent host, certain predisposing factors such as diabetic mellitus, metabolic acidosis, malignancy, corticosteroid treatment, and other situations that lead to dysregulation and exhaustion of immune system can make a host vulnerable.[6] India already bears the brunt of diabetes epidemic with a prevalence of more than 77 million cases according to data provided by International Diabetes Federation in 2020 which has conferred it as the “global capital of diabetes.”[7] For such a country with high prevalence of diabetes and cancer, it is not surprising that India contributes to about 40% of the global burden of mucormycosis with a prevalence of approximately 140 cases per million population.[8]
Careful investigation of the recently reported cases of osteomyelitis of fungal origin involving the craniofacial bones in patients having a history of COVID-19 infection has led to establishment of a strong association exists between COVID-19 infection and mucormycosis.[9] Results from multiple researchers have led to a common inference that the severity of COVID-19 infection also correlates with uncontrolled diabetes mellitus.[10] The present review aims to elucidate the existing relationship between COVID-19, diabetes, and mucormycosis with an objective to make the cascade of events involved in pathogenesis of COVID-19-associated mucormycosis easily understandable by the readers.
ETIOLOGY
Causative agents
In earlier times, the infection was termed as “Zygomycosis” with fungi belonging to the phylum Zygomycetes as causative agents. Zygomycetes are aseptate thermotolerant molds ubiquitously present in the environment typically thriving on decaying matter such as dead leaves, wood, and soil.[11,12] However, the phylum Zygomycetes involve different orders of fungi which cause distinct types of infections, and thus, the terminology was considered to be very broad. Similarly, “Entomophthoromycosis” and “Phycomycosis” have been used synonymously with mucormycosis, but the infections are caused by taxonomically different groups of fungi even though their clinicopathological characteristics overlap.[13] Over the years, the etiological fungi associated with mucormycosis have been methodically narrowed down to certain species belonging to the family Mucoraceae.[14] Among various species, Rhizopus oryzae has been most commonly isolated from patients with mucormycosis infections accounting for 70% of the cases.[15] Other species commonly associated with the infection include Mucor spp. and Lichtheimia spp., followed by less common ones such as Rhizomucor, Actinomucor, Cokeromyces, Cunninghamella, Apophysomyces, Saksenaea, and Syncephalastrum which are found in <5% of cases.[16] The fungi characteristically exhibit aseptate, broad hyphae of dimensions averaging 3–30 μm that branch at right angles. The hyaline appearance of the fungal hyphae on histopathological examination can be attributed to the presence of chitinous component within the fungal wall coupled with lack of pigmentation or septae.[17]
Modes of entry
Despite their ubiquitous presence, the organisms are seldom present among normal commensal microflora of skin or gastrointestinal tract. The fungi are generally incapable of suffusing through intact skin or mucous membranes. Breach in the mucocutaneous integrity because of trauma, wounds, mechanical procedures, or tooth extraction sockets creates passageways for entry of the fungi. In addition, the fungal spores may enter through the nose or may be introduced directly into the oral cavity with the use of contaminated tongue depressors, wooden applicators, surgical dressings, non-sterile adhesive tapes, and other equipment used in dentistry such as forceps, burs, or airotor handpiece.[18,19]
The present COVID-19 pandemic scenario provides further opportunities for entry of the pathogens due to prolonged hospital stays, shortage of health care workers, and subsequent aseptic conditions. The most common mode of entry has been described as through the mechanical ventilators used to deliver oxygen in hypoxemic patients.[20] Direct access into the bloodstream through intravenous catheters or subcutaneous injections used for drug delivery in the treatment of COVID-19 is another possibility.
Risk factors
Once the spores bypass mucocutaneous barriers and manage to enter the body, they are encountered by polymorphonuclear lymphocytes as well as mononuclear phagocytes.[21] Exposure of neutrophils to the fungal hyphae and spores elicits a pro-inflammatory response due to upregulation of Toll-like receptors present on macrophages and dendritic cells with a resultant induction of NF-KB pathway.[22] Consequently, oxidative metabolites and cationic peptides such as defensins are generated; and along with chemotaxis of natural killer cells, these are able to actively destroy the invading pathogens.[21] Besides, the rich vasculature in the maxillofacial region further restricts the incidence of opportunistic infections.[23] In this manner, an immunocompetent host is able to ward off the infection caused by R. oryzae or other related species.
As a result, existence of a condition that compromises the host’s immune system such that it is unable to fight against the fungal invasion is necessitated to establish the infection. The underlying conditions associated with mucormycosis that accounted for majority of the cases were identified as diabetes (36%) and malignancy (17%) in a comprehensive review (n = 929). Other risk factors for mucormycosis include solid organ or bone marrow transplantation, injection drug use, trauma, and burns.[23] Studies have reported that patients with AIDS do not exhibit an increased affinity to develop mucormycosis, thus, suggesting that T-lymphocytes may not play an active role in resistance against the infection.[24] Even so, role of polymorphonuclear leukocytes in combat against mucormycosis is undeniable, and thus, patients suffering from neutropenia or defects in neutrophils are predisposed to develop the infection.[25] Although hematological malignancies are more common in western countries, uncontrolled diabetes and trauma predominate as the fons et origo of mucormycosis in developing countries such as India.[26,27]
PATHOGENESIS
The fungi are of relatively bigger dimensions as compared to other species and thus are easily retained in the paranasal sinuses. However, certain species of small size bodies may also reach the lungs.[28] In the event that the epithelium is disrupted, the fungal spores adhere to the extracellular matrix proteins present in the basement membrane, laminin, and collagen VI by specific binding and subsequently secrete lipolytic/glycosidic enzymes and proteases that degrade the underlying stroma ultimately facilitating fungal invasion into the host tissues.[29,30] The most characteristic process in the pathogenicity of mucormycosis [Figure 1] is the frank angioinvasion by colonies of fungi causing thrombosis of the involved blood vessels resulting in subsequent tissue ischemia and necrosis.[31] The lack of blood supply due to occlusion of blood vessels by the fungi protects the fungi by prevent systemic antifungal drugs and host inflammatory cells from reaching the site of infection.[18]
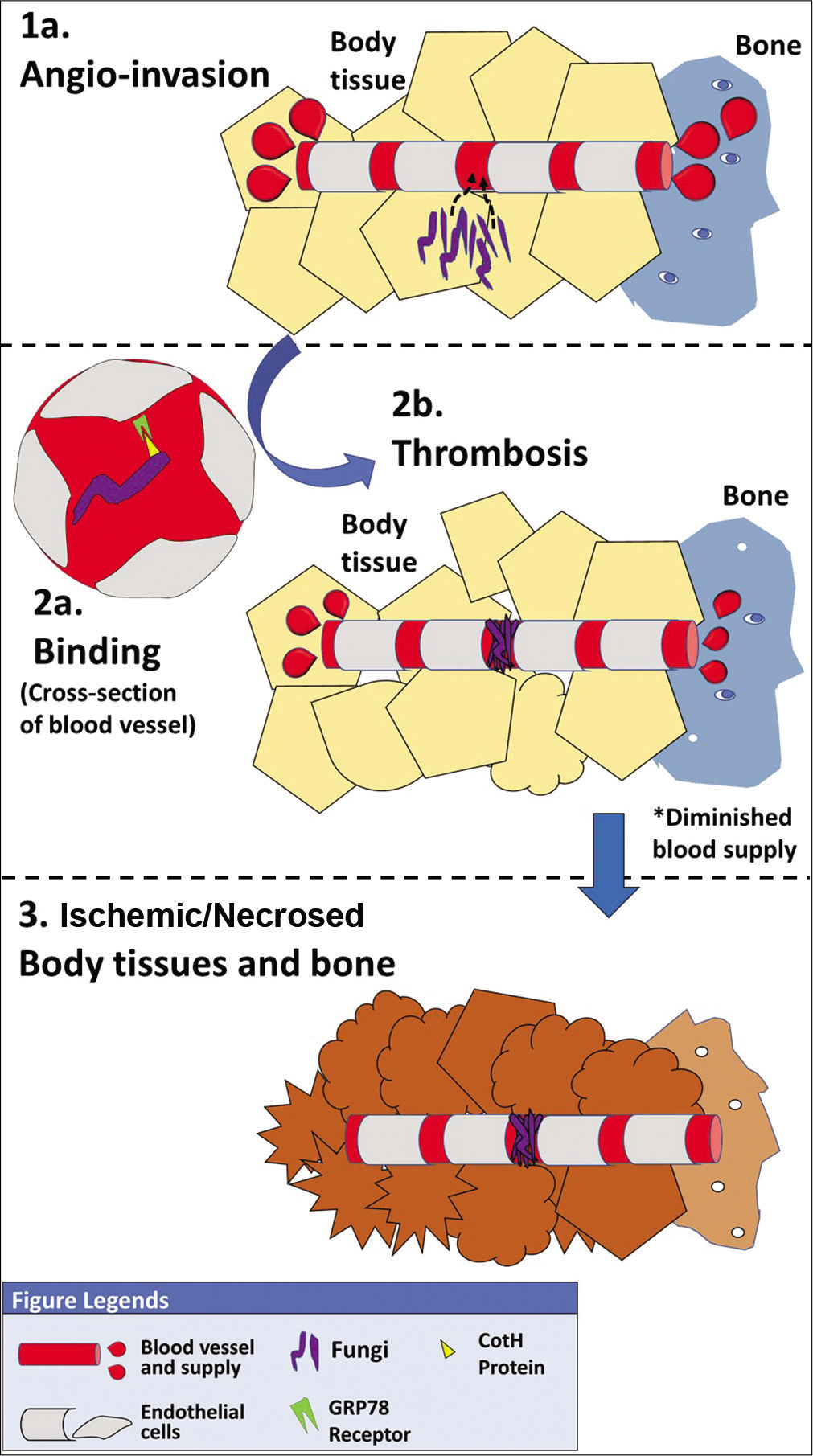
- Pathogenetic process of mucormycosis.
To achieve thrombosis, the fungi must adhere to the endothelial cells and disrupt their integrity so as to gain access to the bloodstream. Mucorales exclusively express spore coat homolog proteins on their surface which enables the spores to effectively bind by means of adhesins to glucose regulator protein 78 (GRP78) receptors present on the endothelial cells.[30] Once bound to the blood vessels, secondary metabolites released during phagocytosis of R. oryzae by the endothelial cells were identified as the cause for disruption of endothelial lining, rather than viable fungi.[32] Platelet-derived growth factor receptor (PDGFR) was shown to permeate endocytosis induce angiogenesis that would subsequently aid in dissemination of the fungi to other organs through bloodstream.[33] Thus, inhibitors to PDGFR could be potential molecules to limit the endothelial damage caused by the fungi and their dissemination.
Another important virulence characteristic noted in Mucorales fungi is the presence of distinct interactions that enable evasion of host defense. Mendelian and epigenetic mutations majorly account for this evasive potential allowing the fungi to evade the inflammatory response by the host and survive in a hostile environment.[34] The fungi are even able to counteract antifungal drugs for which calcineurin has been identified as a virulence factor.[35]
Role of iron
Unlike other opportunistic fungi, R. oryzae require iron for vital processes of the cell contributing to the cell growth and development.[36] Normally, iron is present in the serum of mammals in bound forms such as transferrin, ferritin, and lactoferrin circumvents the effects that may arise due to toxicity of free iron.[37] Therefore, procurement of free iron from the host is a prerequisite for the growth of R. oryzae for which the organism has developed distinct mechanisms.
Fungi inherently secrete low-molecular-weight iron chelators, termed as “siderophores,” that possess affinity for iron surpassing that of transferrin and lactoferrin.[38,39] R. oryzae secretes one such polycarboxylate siderophore known as “rhizoferrin” that acquires iron from host through a receptor-mediated process dependent on energy.[40] However, rhizoferrin has been deemed as inefficient in acquiring iron from the host serum.[41] Consequently, the role of endogenous siderophores in virulence of the organism may be limited which is also highlighted by the fact that it has adapted to utilize xenosiderophores such as deferoxamine.
Deferoxamine is included in the treatment regimen for patients at risk of developing toxicity due to iron overload such as those on renal dialysis. It directly chelates the bound iron in transferrin resulting in iron-ferrioxamine complex which liberates free ferrous iron by reduction at the fungal cell surface through Fob receptors.[42] Compared to other opportunistic fungi such as A. fumigatus or Candida albicans, it has been demonstrated that Rhizopus spp. can acquire 8–40 times the amount of iron through deferoxamine.[43] The ferrous iron is then ingested by the fungus by means of a high-affinity plasma membrane iron permease (FTR1) after reoxidization by copper oxidase.[44] The FTR1 also facilitates release of ferric iron from host hemoglobin through degradation by means of heme oxygenases.[18] Therefore, anti-FTRP1 targeted therapy may play a key role in mitigating mucormycosis infection in future.
The extent of availability of free iron has been directly linked to severity of infection caused by Rhizopus spp. Although deferoxamine in itself is not a pathogenetic factor for the infection, it serves to provide iron to the fungi which was unavailable to it previously. Animal models have also demonstrated that administration of deferoxamine or free iron has reduced the survival of the organism infected with Rhizopus spp.[45] Thus, conditions, such as acidosis that impair iron-binding capacity of transferrin by proton-mediated displacement of ferric ions, tend to create favorable environments in which the fungi thrive.[46] Patients undergoing repeated blood transfusions due to underlying conditions such as myelodysplastic syndrome tend to develop iron overload which predisposes them to mucormycosis.[47]
Modes of presentation
Depending on the areas or organs infected by the fungi, various clinical types of mucormycosis have been observed such as rhinocerebral, pulmonary, cutaneous, gastrointestinal, and disseminated forms. These forms obviously correspond to the mode of entry and other pathoanatomical factors. Since the spores are generally acquitted through inhalation, rhinocerebral is the most common type observed clinically (39%) followed by pulmonary infection (24%). Gastrointestinal type is observed in patients with malnutrition and cutaneous type is observed in those having history of trauma or burns. In established cases, wherein the pathogen has managed to invade the blood vessels and spread to other organs, disseminated type of infection is noted.[12,18,48] The clinical types were also shown to be related with the patient’s underlying conditions as a reflection of the host’s immune function and other related factors. For instance, patients with malignancy tend to develop pulmonary infections while those suffering from uncontrolled diabetes present with sino-orbital or rhinocerebral forms.[23]
In a long-term analysis of mucormycosis cases in India (n = 372), rhino-orbito-cerebral involvement was found to be most frequent (44.2%).[49] Considering the fact that trauma is the most common predisposing factor for mucormycosis, second to diabetes in India, the corresponding cutaneous type was found to be relatively more common (15.5%). An upsurge in the incidence rate of mucormycosis cases in India was reported in the past two decades that could be attributed primarily to the rising epidemic of diabetes. In addition, the relatively hot and humid climate in most parts of the country along with a significant portion of malnourished population produce optimal settings for the fungi to thrive and contribute to its incidence.[8,49]
In contrast to other common fungal infections such as Candidiasis (20–50%) and Aspergillosis (35–45%), the mortality rate of mucormycosis is higher ranging from 50% to 100% depending on the clinical type.[15,23] The mortality rate is even higher for patients with underlying conditions such as diabetes mellitus or those with extensive trauma and burns. Disseminated forms of the disease are almost inevitably fatal REF. Naturally, rhino-occulo-cerebral forms have higher mortality rate than early cases limited to rhinomaxillary area. The alarmingly high mortality rates indicate that the disease cannot be trifled with and warrants early diagnosis and reversal of the condition before it progresses to the disseminated form.
Implications of diabetes
Diabetes and mucormycosis are almost inseparable considering the fact that majority of the reported cases have an underlying history of diabetes mellitus. Diabetic ketoacidosis (DKA) hinders the ability of phagocytes and polymorphonuclear leukocytes to effectively respond to the infection.[50] The systemic acidosis significantly increases the proportion of free iron available in the serum by releasing it from the binding proteins, thus, creating a rich source of nutrition for the fungi.[21] Furthermore, Rhizopus arrhizus also produce the enzyme ketoreductase by means of which the fungi can utilize ketone bodies available in the serum of a patient with DKA to enhance their growth.[51] As mentioned previously, the expression of GRP78 receptors present on the surface of endothelial cells, that facilitate angioinvasion, is also increased in the sinus, lungs, and brain of individuals with DKA.[40] Overall, the defective function of immunomodulatory cells, increase in availability of free iron, and increased expression of virulence factors account for higher morbidity and mortality in mucormycosis patients with diabetes mellitus [Figure 2]. The dreadful combination is even higher in India which comprises a significant portion of diabetic individuals.
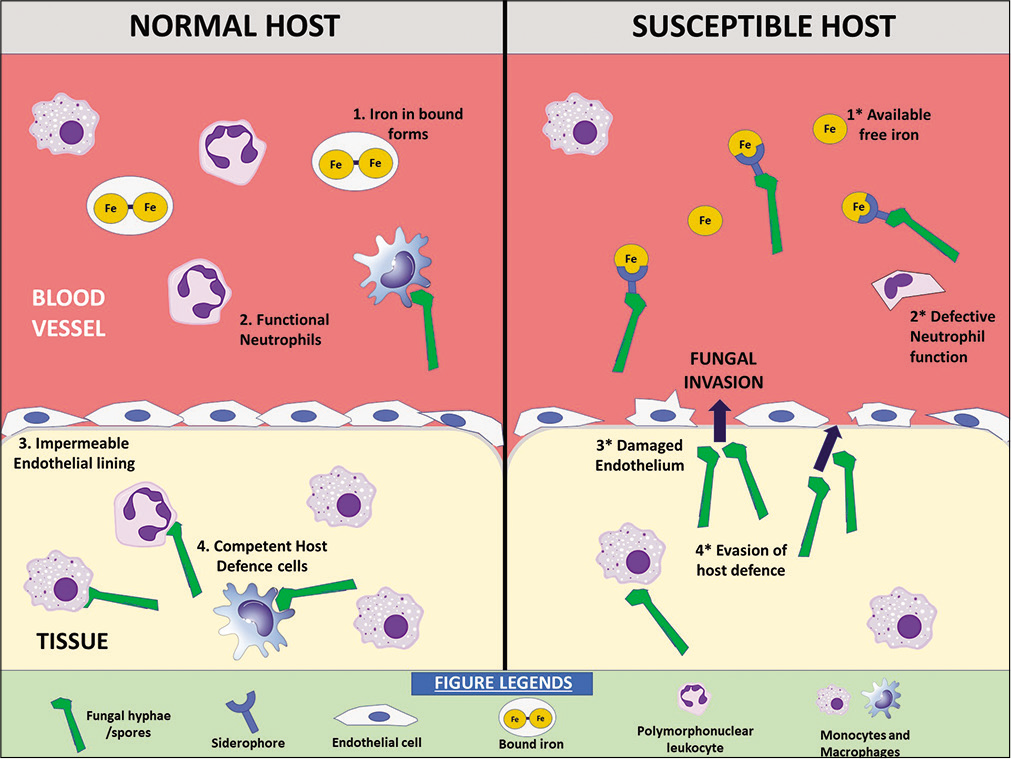
- Factors predisposing to mucormycosis in a susceptible host.
Implications of COVID-19
Besides claiming millions of lives worldwide by itself, the aftereffects of COVID-19 infection and its treatment have created a unique scenario that is in favor of mucormycosis. Umpteen cases of fungal osteomyelitis developing in patients previously diagnosed with COVID-19 have come into light in recent times and have been the topic of interest for various academic groups as well as online and offline media.[9] The presentation of these cases, although anecdotal, has more or less been identical to the rhinomaxillary form of mucormycosis.[52]
Current guidelines have recommended use of remdesivir and glucocorticoids in treatment regimen of COVID-19 patients, especially in those requiring oxygen support.[53] Although the drugs are effective in resolving the hypoxemic condition of the patients, the fact that they create dysregulation of immune system cannot be overlooked. Glucocorticoids add to the already existing glycemic load in diabetic patients, consequently worsening their hyperglycemia and DKA.
In addition, the SARS-CoV-2 virus in itself is able to cause impairment of cell-mediated immune response by reducing the count of T lymphocytes.[3] Acute respiratory dysfunction syndrome and multiorgan dysfunction linked with cytokine storm further add to compromise of the immune capability of the patients suffering from severe forms of COVID-19. Overall, a combination of multiple associated factors constructs a cascade of events that ultimately cause immune exhaustion and predispose the patient to secondary bacterial and fungal infections [Figure 3].
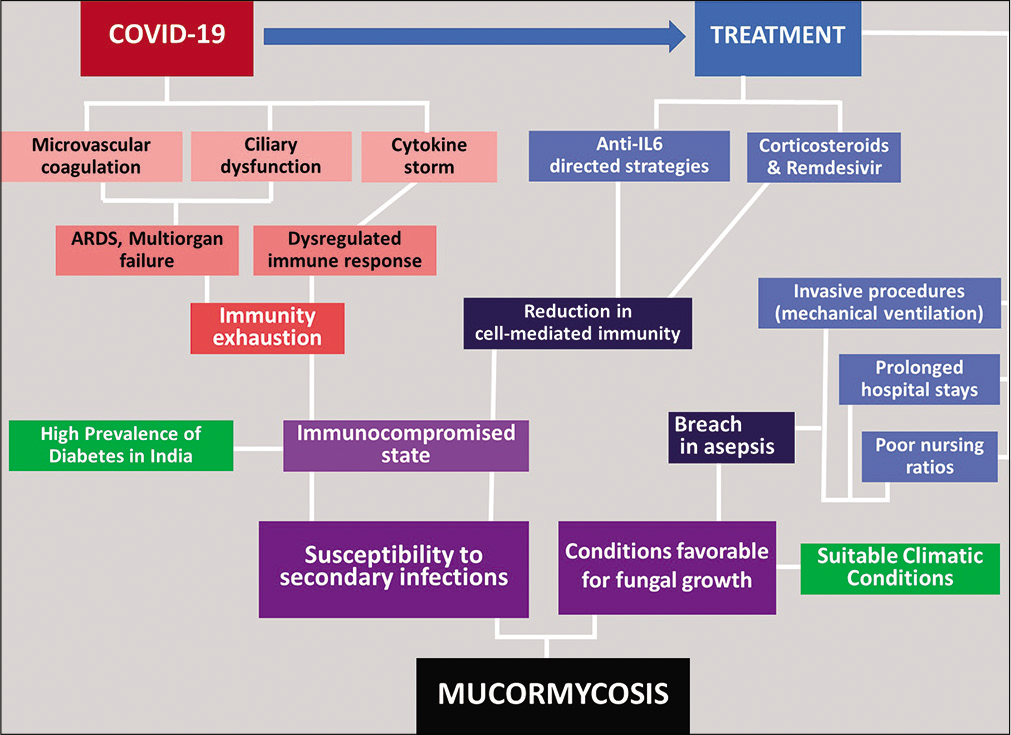
- Cascade of events in COVID-associated mucormycosis.
The hospitals are pre-occupied with booming number of cases of COVID-19 and majority of health-care resources are shifted to combat the perilous effects of the pandemic which might account for delay in diagnosis of mucormycosis cases. Since the organism possesses virulence factors that enable it to grow and disseminate very rapidly, the “window of opportunity” to diagnose and resolve the condition is extremely short, particularly in cases of COVID-19 patients. Delay in commencement of therapy has been reported to result in a doubled mortality rate in patients with mucormycosis infection.[54]
The mortality in cases of mucormycosis associated with COVID-19 patients has been reported as much higher (87.5%).[5] However, patients may refrain from reporting to the hospitals post-recovery from COVID-19 due to psychological or financial reasons, and thus, the actual mortality rate of COVID-associated mucormycosis might be much higher.[55]
CONCLUSION
The dreadful disease of mucormycosis has always been a cause of concern in India with high prevalence and mortality rates due to high prevalence of diabetes and suitable climatic conditions throughout the country. The addition of COVID-19 pandemic to the cauldron of existing diabetes and mucormycetes misfortune has created an ultimate recipe of morbidity and mortality in India. The recent and still ongoing rise in number of reported cases of COVID-associated mucormycosis warrants more attention of medical professionals, especially dental surgeons, since they would be most efficiently able to identify the disease in an early stage, minimizing the ensuing damage. A thorough understanding of pathogenesis of the disease would further augment the ability of health-care facilities and workers to develop precautionary and prophylactic measures against the developing malice.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- WHO Coronavirus (COVID-19) Dashboard. 2021. Geneva: World Health Organization; Available from: https://covid19.who.int [Last accessed on 2021 May 13]
- [Google Scholar]
- Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: A meta-analysis. JAMA. 2020;324:1330-41.
- [CrossRef] [PubMed] [Google Scholar]
- Invasive fungal diseases during COVID-19: We should be prepared. J Mycol Med. 2020;30:100971.
- [CrossRef] [PubMed] [Google Scholar]
- Infections due to emerging and uncommon medically important fungal pathogens. Clin Microbiol Infect. 2004;10(Suppl 1):48-66.
- [CrossRef] [PubMed] [Google Scholar]
- Coronavirus disease (Covid-19) associated mucormycosis (CAM): Case report and systematic review of literature. Mycopathologia. 2021;186:289-98.
- [CrossRef] [PubMed] [Google Scholar]
- Infections in immunocompromised patients from diabetes mellitus. Int J Healthc Sci. 2017;5:583-7.
- [Google Scholar]
- International Diabetes Federation SEA Members. 2021. Available from: https://www.idf.org/our-network/regions-members/south-east-asia/members/94-india.html [Last accessed on 2021 May 13]
- [Google Scholar]
- Epidemiology of mucormycosis in India. Microorganisms. 2021;9:523.
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19 and mucormycosis of the craniofacial skeleton: Causal, contributory or coincidental? J Maxillofac Oral Surg. 2021;20:1-2.
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19 and diabetes mellitus: From pathophysiology to clinical management. Nat Rev Endocrinol. 2021;17:11-30.
- [CrossRef] [PubMed] [Google Scholar]
- Mucormycoses in patients with hematologic malignancies: An emerging fungal infection. Haematologica. 2005;90(Suppl):ECR22.
- [Google Scholar]
- Disseminated Rhizopus microsporus infection following allogeneic hematopoietic stem cell transplantation in a child with severe aplastic anemia. Transpl Infect Dis. 2013;15:E216-23.
- [CrossRef] [PubMed] [Google Scholar]
- Taxonomy of fungi causing mucormycosis and entomophthoramycosis (zygomycosis) and nomenclature of the disease: Molecular mycologic perspectives. Clin Infect Dis. 2012;54(Suppl 1):S8-15.
- [CrossRef] [PubMed] [Google Scholar]
- Zygomycetes in human disease. Clin Microbiol Rev. 2000;13:236-301.
- [CrossRef] [PubMed] [Google Scholar]
- Novel perspectives on mucormycosis: Pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18:556-69.
- [CrossRef] [PubMed] [Google Scholar]
- Mucormycosis caused by unusual mucormycetes, non-rhizopus,-mucor, and-lichtheimia species. Clin Microbiol Rev. 2011;24:411-45.
- [CrossRef] [PubMed] [Google Scholar]
- Chitin: A “hidden figure” in the fungal cell wall. The Fungal Cell Wall In: Cham Springer. 2019. p. :83-111.
- [CrossRef] [PubMed] [Google Scholar]
- Pathogenesis of mucormycosis. Clin Infect Dis. 2012;54(Suppl 1):S16-22.
- [CrossRef] [PubMed] [Google Scholar]
- Cross contamination in dentistry: A comprehensive overview. J Educ Ethics Dent. 2012;2:3-9.
- [CrossRef] [Google Scholar]
- Secondary infection with rhino-orbital cerebral mucormycosis associated with COVID-19. Orbit. 2021;2021:1-4.
- [CrossRef] [PubMed] [Google Scholar]
- Zygomycetes hyphae trigger an early, robust proinflammatory response in human polymorphonuclear neutrophils through toll-like receptor 2 induction but display relative resistance to oxidative damage. Antimicrob Agents Chemother. 2008;52:722-4.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology and outcome of zygomycosis: A review of 929 reported cases. Clin Infect Dis. 2005;41:634-53.
- [CrossRef] [PubMed] [Google Scholar]
- Agents of mucormycosis and related species In: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practice of Infectious Diseases (6th ed). Philadelphia, PA: Elsevier; 2005. p. :2979.
- [Google Scholar]
- Generation of chemotactic factors by Rhizopus oryzae in the presence and absence of serum: Relationship to hyphal damage mediated by human neutrophils and effects of hyperglycemia and ketoacidosis. Infect Immun. 1982;38:1123-9.
- [CrossRef] [PubMed] [Google Scholar]
- Mucormycosis and entomophthoramycosis: A review of the clinical manifestations, diagnosis and treatment. Clin Microbiol Infect. 2004;10(Suppl 1):31-47.
- [CrossRef] [PubMed] [Google Scholar]
- The rising trend of invasive zygomycosis in patients with uncontrolled diabetes mellitus. Med Mycol. 2006;44:335-42.
- [CrossRef] [PubMed] [Google Scholar]
- Mucormycosis-from the pathogens to the disease. Clin Microbiol Infect. 2014;20(Suppl 6):60-6.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular cloning of an extracellular aspartic proteinase from Rhizopus microsporus and evidence for its expression during infection. Med Mycol. 2002;40:61-71.
- [CrossRef] [PubMed] [Google Scholar]
- Characterization of an extracellular subtilisin protease of Rhizopus microsporus and evidence for its expression during invasive rhinoorbital mycosis. Med Mycol. 2006;44:723-31.
- [CrossRef] [PubMed] [Google Scholar]
- A clinicopathological study of pulmonary mucormycosis in cancer patients: Extensive angioinvasion but limited inflammatory response. J Infect. 2009;59:134-8.
- [CrossRef] [PubMed] [Google Scholar]
- Rhizopus oryzae adheres to, is phagocytosed by, and damages endothelial cells in vitro. Infect Immun. 2005;73:778-83.
- [CrossRef] [PubMed] [Google Scholar]
- An integrated genomic and transcriptomic survey of mucormycosis-causing fungi. Nat Commun. 2016;7:12218.
- [CrossRef] [PubMed] [Google Scholar]
- Antifungal drug resistance evoked via RNAi-dependent epimutations. Nature. 2014;513:555-8.
- [CrossRef] [PubMed] [Google Scholar]
- Calcineurin orchestrates dimorphic transitions, antifungal drug responses and host-pathogen interactions of the pathogenic mucoralean fungus Mucor circinelloides. Mol Microbiol. 2015;97:844-65.
- [CrossRef] [PubMed] [Google Scholar]
- Acquisition, transport, and storage of iron by pathogenic fungi. Clin Microbiol Rev. 1999;12:394-404.
- [CrossRef] [PubMed] [Google Scholar]
- A mechanism of susceptibility to mucormycosis in diabetic ketoacidosis: Transferrin and iron availability. Diabetes. 1982;31:1109-14.
- [CrossRef] [PubMed] [Google Scholar]
- Iron uptake in fungi: a system for every source. Biochim Biophys Acta. 2006;1763:636-45.
- [CrossRef] [PubMed] [Google Scholar]
- Nutritional immunity: Transition metals at the pathogen-host interface. Nat Rev Microbiol. 2012;10:525-37.
- [CrossRef] [PubMed] [Google Scholar]
- The endothelial cell receptor GRP78 is required for mucormycosis pathogenesis in diabetic mice. J Clin Invest. 2010;120:1914-24.
- [CrossRef] [PubMed] [Google Scholar]
- Host defenses against zygomycetes. Clin Infect Dis. 2012;54(Suppl 1):S61-6.
- [CrossRef] [PubMed] [Google Scholar]
- Fob1 and Fob2 proteins are virulence determinants of Rhizopus oryzae via facilitating iron uptake from ferrioxamine. PLoS Pathog. 2015;11:e1004842.
- [CrossRef] [PubMed] [Google Scholar]
- Mucormycosis during deferoxamine therapy is a siderophore-mediated infection. In vitro and in vivo animal studies. J Clin Invest. 1993;91:1979-86.
- [CrossRef] [PubMed] [Google Scholar]
- The high affinity iron permease is a key virulence factor required for Rhizopus oryzae pathogenesis. Mol Microbiol. 2010;77:587-604.
- [CrossRef] [PubMed] [Google Scholar]
- Iron source preference and regulation of iron uptake in Cryptococcus neoformans. PLoS Pathog. 2008;4:e45.
- [CrossRef] [PubMed] [Google Scholar]
- Iron acquisition: A novel perspective on mucormycosis pathogenesis and treatment. Curr Opin Infect Dis. 2008;21:620-5.
- [CrossRef] [PubMed] [Google Scholar]
- Mucormycosis in allogeneic bone marrow transplant recipients: Report of five cases and review of the role of iron overload in the pathogenesis. Bone Marrow Transplant. 1999;24:307-12.
- [CrossRef] [PubMed] [Google Scholar]
- A global analysis of mucormycosis in France: The Retrozygo study (2005-2007) Clin Infect Dis. 2012;54(Suppl 1):S35-43.
- [CrossRef] [PubMed] [Google Scholar]
- Mucormycosis in India: Unique features. Mycoses. 2014;57:85-90.
- [CrossRef] [PubMed] [Google Scholar]
- Mucormycosis in a diabetic ketoacidosis patient. Asian Pac J Trop Biomed. 2013;3:830-3.
- [CrossRef] [Google Scholar]
- Ulcerative vesicular and bullous lesions In: Greenberg MS, Glick M, eds. Burket's Oral Medicine Diagnosis and Treatment. Amsterdam, Netherlands: Elsevier; 2003. p. :79.
- [Google Scholar]
- Therapeutic Management of Adults with COVID-19. Available from: https://www.covid19treatmentguidelines.nih.gov/therapeutic-management [Last accessed on 2021 May 15]
- [Google Scholar]
- Acute invasive rhino-orbital mucormycosis in a patient with COVID-19-associated acute respiratory distress syndrome. Ophthalmic Plast Reconstr Surg. 2021;37:e40.
- [CrossRef] [PubMed] [Google Scholar]
- Rapid assessment of psychological and epidemiological correlates of COVID-19 concern, financial strain, and health-related behavior change in a large online sample. PLoS One. 2020;15:e0241990.
- [CrossRef] [PubMed] [Google Scholar]






