Evaluation of genetic polymorphism of interleukin-1β (rs16944) in chronic and aggressive periodontitis in a group of the Bengali population of West Bengal, India

*Corresponding author: Avishek Das, Department of Periodontics, Dr. R Ahmed Dental College and Hospital, 114, AJC Bose Road, Kolkata - 700014, West Bengal, India. avidoc2010@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Das A, Kundu R, Pal AK, Bagchi S. Evaluation of genetic polymorphism of interleukin-1β (rs16944) in chronic and aggressive periodontitis in a group of the Bengali population of West Bengal, India. J Global Oral Health 2021;4(1):27-32.
Abstract
Objectives:
The genetic basis of periodontitis was demonstrated by formal genetic studies which were focused on a range of various candidate genes selected for their roles in the immune system like genes of the interleukins (ILs) which regulate the intensity of host immunity-inflammatory response. This regulation of host response may be associated with the genetic polymorphisms, specific single-nucleotide polymorphisms of the genes of various ILs. Interleukin-1 (IL-1) is a principal mediator of inflammatory responses acting on many cell types and is itself produced by many different cells, including macrophages, endothelial cells, B cells, fibroblasts, epithelial cells, astrocytes, and osteoblasts in response to microorganisms, bacterial toxins, and complement components. In the present study, an attempt has been made to explore the role of IL-1β-511 (rs16944) genetic polymorphism in chronic as well as aggressive periodontitis in a group of the Bengali population of West Bengal, India.
Materials and Methods:
A total of 88 Bengali patients of both sexes were recruited in this study and they were divided into three groups: Group A (chronic periodontitis group), Group B (aggressive periodontitis group), and Group C (healthy control). The clinical parameters taken into consideration in the assessment of chronic and aggressive periodontitis were plaque index, calculus index, gingival index, probing pocket depth (PPD), and clinical attachment loss (CAL). A 3 ml of peripheral venous blood was collected from each selected participant and transferred to 3% EDTA containing serum vial and stored at −20°C for DNA extraction. DNA extraction was performed by the phenol chloroform method and ethanol precipitation. Genotyping of extracting DNA samples was carried out for locus IL-1β-511 (rs16944) by real-time polymerase chain reaction. Hardy–Weinberg equilibrium was tested for the gene polymorphism and association between genotypes and cases was examined by odds ratio with 95% confidence interval (CI) and Chi-square analysis using R programming software. Allelic frequencies were calculated according to the number of different alleles observed and the total number of alleles examined. Statistical significance was defined as P < 0.05.
Results:
On analysis of minor allele frequencies of total periodontitis cases and control, the results were found to be statistically insignificant with P = 0.9799. Minor allele frequency of overall periodontitis cases and controls was 0.405 and 0.403, respectively; the odds ratio was 1.008 and 95% CI ranges from 0.53 to 1.88.
Conclusion:
The present study suggested no association of single-nucleotide polymorphism of IL-1β-511 (rs16944) with total periodontitis cases (both chronic periodontitis and aggressive periodontitis) in the present study cohort.
Keywords
Genotyping
Interleukin
Minor allele frequency
Real-time polymerase chain reaction
Single-nucleotide polymorphism
INTRODUCTION
In subjects susceptible to periodontitis, commonly an imbalance exists in between the host’s immune system and the oral bacteria.[1,2] Cytokines are regulators of host responses to infection, immune responses, inflammation, and trauma.[3] The prevalence of chronic periodontitis varies among various races. The prevalence of severe periodontitis among participants aged approximately 40–50 years and the estimates were 21% in Germany and 16%, 28%, and 32% in various populations from the United States.[4] In Indian population, the prevalence rate of moderate chronic periodontitis is 17.5% in 35–44 years age group and 21.4% in 65–74 years age group.[5] The prevalence of loss of attachment of >3 mm in the 44 years age group is highest in Maharashtra (78%) followed by Orissa (68%) and Delhi (46%).[5] Genetic research can improve the understanding of the factors that mediate the immune response and explain why this response often greatly differs between individuals who have the same environmental context and comparable lifestyle habits. The first ever study investigating the relationship between IL-1β C-511T polymorphism and chronic periodontitis, demonstrated no significant association between IL-1β C-511T polymorphism and chronic periodontitis was detected.[6] However, another study demonstrated that allele T for IL-1β (C-511T) associated with chronic periodontitis in a subgroup of Afro-Americans and mulattos.[7] A meta-analysis was performed to investigate the association of the IL1α-889, IL1β+3954, IL1β-511, TNF-α-308, and IL6-174 polymorphisms in aggressive periodontitis and chronic periodontitis.[8] They did not find any associations for aggressive periodontitis, but moderate and weak positive associations were found for the IL1 composite and IL1β-511 genotypes, respectively, in chronic periodontitis patients. In the present study, an attempt has been made to explore the role of IL-1β-511 (rs16944) gene polymorphisms in chronic periodontitis (CP) and aggressive periodontitis (AgP) in a group of the Bengali population of West Bengal, India.
MATERIALS AND METHODS
The study was conducted between February 2017 and September 2018. A total of 88 Bengali participants (both male and female) with age between 30 and 55 years were selected from the OPD of the Department of Periodontics of Dr. R. Ahmed Dental College and Hospital, Kolkata, and they were divided into three groups. Group A comprised 49 CP patients; Group B comprised 8 AgP patients, and Group C comprised 31 healthy volunteers. Diagnosis of chronic periodontitis (CP) and aggressive periodontitis (AgP) was made as per AAP 1999 classification of periodontal diseases. Smokers, pregnant females, patients with some other systemic diseases, and patients on antibiotics or NSAIDs therapy or any periodontal therapy within the past 6 months were excluded from the study. The clinical parameters that were considered in the assessment of CP and AgP are plaque index (Silness and Löe, 1964),[9] calculus index (calculus component of oral hygiene index-simplified, Greene and Vermillion, 1964),[10] gingival index (Löe and Sillness, 1963),[11] probing pocket depth (PPD), and clinical attachment loss (CAL). The inclusion criteria of all clinical parameters that had been used for assessing chronic periodontitis patients (Group A) were PPD (mm) ≥5 mm , CAL ≥ 3 mm, plaque index ≥2, gingival index ≥2, calculus index ≥2, and radiographic evidence of bone loss. Patients having rapid loss of attachment and tooth-supporting bone, otherwise healthy (i.e., not suffering from any systemic disease or condition that could be responsible for the present periodontitis), presence of familiar aggregation along with secondary features like inconsistent presence of etiologic factors, and typical radiographic bone destruction pattern (arc shaped) in molar-incisor group (AAP 1999 classification of periodontal diseases) considered as aggressive periodontitis case in Group B. Volunteers (age and sex matched) with healthy gingiva having PPD of 0–3 mm, no evidence of clinical attachment loss, no evidence of radiographic bone loss, no signs of gingival inflammation, and not suffering from any systemic disease considered as healthy controls (Group C). The present study was conducted in accordance with the Declaration of Helsinki and received approval from the Institutional Review Board of Dr. R. Ahmed Dental College and Hospital (IRB No. DCH/73/8-19) and the written informed consent was obtained from all patients.
Sample collection
A 3 ml of peripheral venous blood was collected from each selected participant and transferred to 3% EDTA containing serum vial and immediately transferred to Human Genetics Unit of Indian Statistical Institute, Kolkata, to store at −20°C for DNA extraction.
DNA extraction from blood sample
DNA extraction was performed by the phenol chloroform method and ethanol precipitation. A 3 ml of whole blood was placed in a 15 ml Falcon tube. A 12 ml reagent A (0.01 M Tris-HCL, pH 7.4, 320 mm sucrose, 5 mm MgCl2, 1% Triton X-100) was added into it for red cell lysis. The above mixture was then placed on a rotating blood mixer for 5 min at room temperature for twice. The whole mixture was centrifuged at 3000 g for 5 min at room temperature for twice. The supernatant was discarded without disturbing cell pellet. Remaining moisture was removed by inverting the tube and blotting onto tissue paper. Then, 1 ml reagent B (0.4 m Tris-HCL, 150 mm NaCl, 0.06 M EDTA, 1% sodium dodecyl sulfate, pH 8.0) was added to cell lysis. Then, vortexed briefly to suspend the cell pellet and added 250 μL of 5M sodium perchlorate and mixed by inverting the tube several times. Then, the tube was placed in a water bath for 30 min. Then, allowed to cool at room temperature and 2 ml ice-cold chloroform was added and mixed in a rotating mixer for 30–60 min. After that, the tube was then centrifuged at 3000 g for 5 min. After centrifugation, the upper phase of the tube was transferred into a clean Falcon tube using a sterile pipette. Then, 2–3 ml ice-cold ethanol was added and inverted the tube gently to allow DNA to precipitate. The DNA then spooled onto the hooked end using a freshly prepared flamed Pasteur pipette. Then, the DNA sample was transferred to a 1.5 ml Eppendorf tube and allowed to air dry. Finally, the sample was suspended in 200 μl TE buffer.
Genotyping
Genotyping of extracted DNA samples was carried out for locus IL-1β-511(rs16944) using 7900 HT (high throughput) fast real-time PCR system instrument (Applied Biosystems, USA) using allele specific TaqMan minor groove binder probe labeled with fluorescent dyes fluorescein amidites and VIC (Life Technologies), according to the manufacturer’s protocols.
Hardy–Weinberg equilibrium was tested for gene polymorphism and association between genotypes and cases (Groups A and B) was examined by the odds ratio with 95% confidence interval (CI) and Chi-square analysis using R programming software (R version 4.0.2). Allelic frequencies were calculated according to the number of different alleles observed and the total number of alleles examined. Statistical significance was defined as P < 0.05.
RESULTS
The study population of 88 participants comprised 57 cases which include 26 males and 31 females among 31 controls (Group C) including 15 males and 16 females. Group A comprised 20 males and 29 females with an average age of 41.43 years (range 24–55 years) with the standard deviation of 8.06. Group B comprised 6 males and 2 females with an average age of 29.44 years (range 18–50 years) with the standard deviation of 11.02. Group C comprised 16 males and 15 females with an average age of 32.48 years (range 22–45 years) with the standard deviation of 6.09. The distribution of the sampled population according to the clinical parameters is shown in [Tables 1 and 2].
| Plaque index (Mean±SD) | Gingival index (Mean±SD) | Calculus index (Mean±SD) | PPD (mm) (Mean±SD) | CAL (mm) (Mean±SD) | |
|---|---|---|---|---|---|
| Group A | 2.20±0.41 | 2.1±0.63 | 2.49±0.51 | 6.10±0.84 | 3.75±0.72 |
| Group C | 0.65±0.49 | 1.25±0.46 | 0.42±0.50 | 1.87±0.71 | 0.52±0.51 |
| Plaque index (Mean±SD) | Gingival index (Mean±SD) | Calculus index (Mean±SD) | PPD (mm) (Mean±SD) | CAL (mm) (Mean±SD) | |
|---|---|---|---|---|---|
| Group B | 1.5±0.53 | 1.75±0.63 | 1.63±0.52 | 6.38±1.06 | 6.88±1.13 |
| Group C | 0.65±0.49 | 1.25±0.46 | 0.42±0.50 | 1.87±0.71 | 0.52±0.51 |
Comparison of allele frequencies and genotype frequencies of IL1β-511 [rs16944] in cases and controls
The distributions of A and G alleles for IL-1β-511 (rs16944) in the group with chronic periodontitis (Group A) and control group (Group C) showed a higher frequency (61%) of the A allele and comparatively lower frequency (39%) of G allele in patients with chronic periodontitis. Similarly, in the control group (Group C), the frequency of A allele was found to be higher (59%) than G allele (41%). The distribution of A and G alleles showed no statistically significant difference between Group A and Group C (P = 0.6843) [Table 3 and Figure 1].
| Groups | Allele counts (frequency %) | Chi-square test (P value) | |
|---|---|---|---|
| A | G | ||
| Chronic periodontitis (Group A) | 60 (61) | 38 (39) | P= 0.6843* |
| Healthy controls (Group C) | 37 (59) | 25 (41) | |
*Statistically insignificant P value

- Allele frequencies of IL-1β (rs16944) single-nucleotide polymorphisms in chronic periodontitis patients versus healthy controls.
Higher frequency of AG heterozygous genotype (70%) for IL-1β-511 (rs16944) has been found in chronic periodontitis cases (Group A) as compared to AA (26%) and GG (4%) genotypes. Similarly, in the control group (Group C), the frequency of the AG genotype was found to be higher (61%) than AA genotype (29%) and GG genotype (10%). The difference between genotype frequencies among Group A and Group C was statistically insignificant (P = 0.0729) [Table 4 and Figure 2].
| Groups | Genotype counts (frequency %) | Chi-square test (P value) | ||
|---|---|---|---|---|
| AA | AG | GG | ||
| Chronic periodontitis (Group A) | 13 (26) | 34 (70) | 2 (4) | 0.0729* |
| Healthy control (Group C) | 9 (29) | 19 (61) | 3 (10) | |
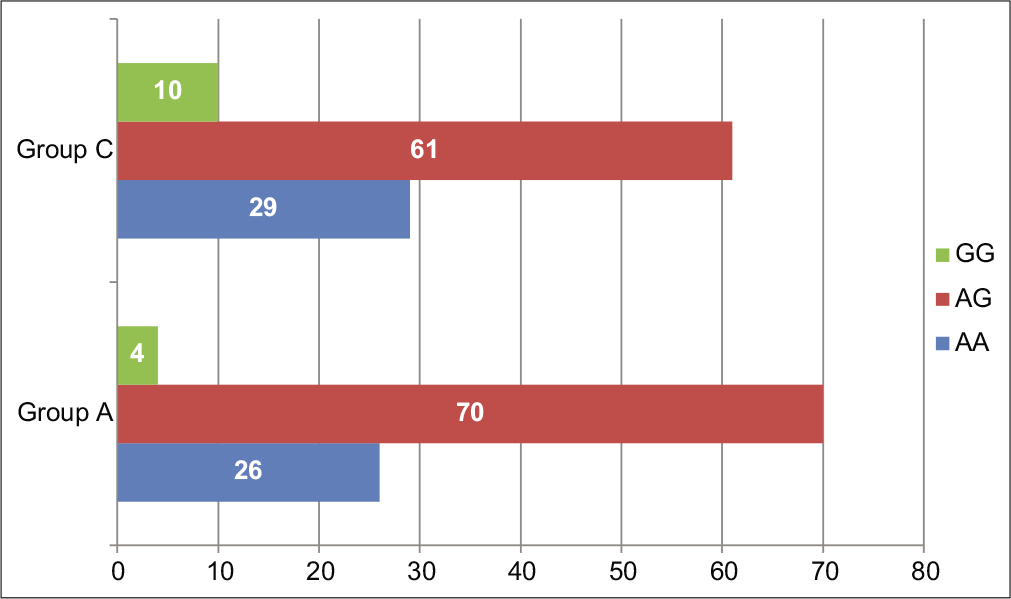
- Genotype frequencies of IL-1β-511 (rs16944) single-nucleotide polymorphisms in chronic periodontitis patients versus healthy controls.
The distributions of A and G alleles for IL-1β-511 (rs16944) between the groups with aggressive periodontitis (Group B) and control group (Group C) showed equal frequency of the A allele (50%) and G allele (50%), which achieved a statistically insignificant result with P = 0.2560, whereas higher frequency of A allele (59%) and comparatively lower frequency of G allele (41%) were seen in Group C. The difference between allele frequencies among Group A and Group C was statistically insignificant (P = 0.2560) [Table 5 and Figure 3].
| Groups | Allele counts (Frequency %) | Chi-square test (P value) | |
|---|---|---|---|
| A | G | ||
| Aggressive periodontitis (Group B) | 8 (50) | 8 (50) | P=0.2560* |
| Healthy control (Group C) | 37 (59) | 25 (41) | |
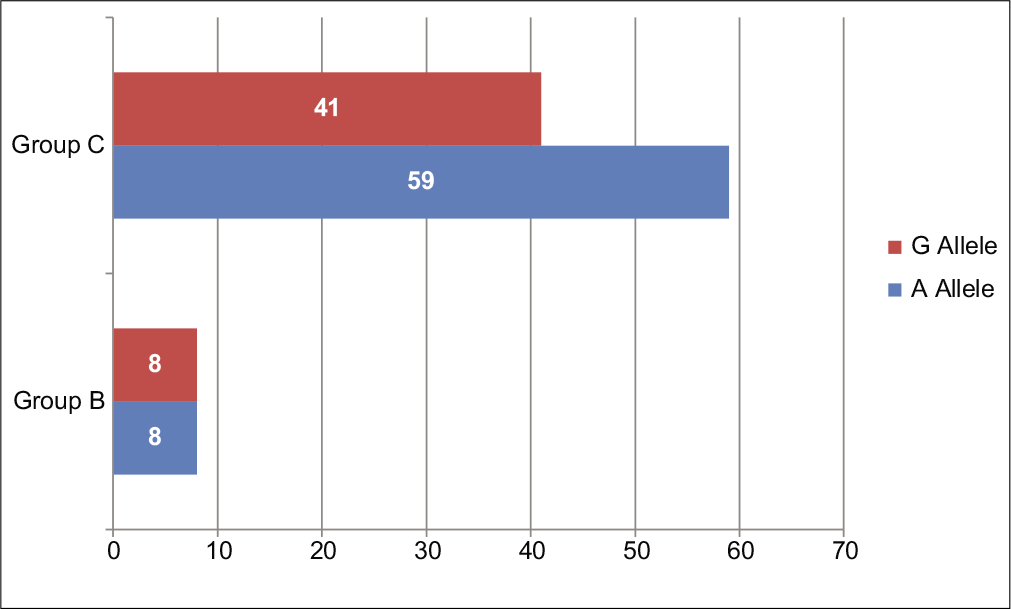
- Allele frequencies of IL-1β-511 (rs16944) single-nucleotide polymorphisms in aggressive periodontitis patients versus healthy controls.
Only AG genotype was found (100%) for IL-1β-511 (rs16944) in aggressive periodontitis cases (Group B), whereas higher frequency of AG (61%) and comparatively lower frequency of AA (29%) and GG (10%) were found in healthy controls (Group C). When compared with the genotype frequency of the healthy control group, a statistically significant result was achieved with P < 0.0001 [Table 6 and Figure 4].
| Groups | Genotype counts (Frequency %) | Chi-square test (P value) | ||
|---|---|---|---|---|
| AA | AG | GG | ||
| Aggressive periodontitis (Group B) | - | 8 (100) | - | <0.0001 |
| Healthy control (Group C) | 9 (29) | 19 (61) | 3 (10) | |

- Genotype frequencies of IL-1β-511 (rs16944) single-nucleotide polymorphisms in aggressive periodontitis patients versus healthy controls.
As the sample size of Group B was too small as compared to other groups, for the analysis of minor allele frequency, both the periodontitis cases (chronic and aggressive periodontitis) have considered as a unified group of periodontitis. On analysis of minor allele frequencies of total periodontitis cases and control, the results were found to be statistically insignificant with P = 0.9799. Minor allele frequency of overall periodontitis cases and controls was 0.405 and 0.403, respectively. The odds ratio was 1.008 and 95% CI ranges from 0.53 to 1.88 [Table 7 and Figure 5].
| Gene | Periodontitis cases | Healthy controls | Minor allele frequency (case) | Minor allele frequency (control) | Odds ratio | 95% confidence interval | P value |
|---|---|---|---|---|---|---|---|
| IL-1β-511 (rs16944) | 57 | 31 | 0.405 | 0.403 | 1.008 | 0.53–1.88 | 0.9799* |
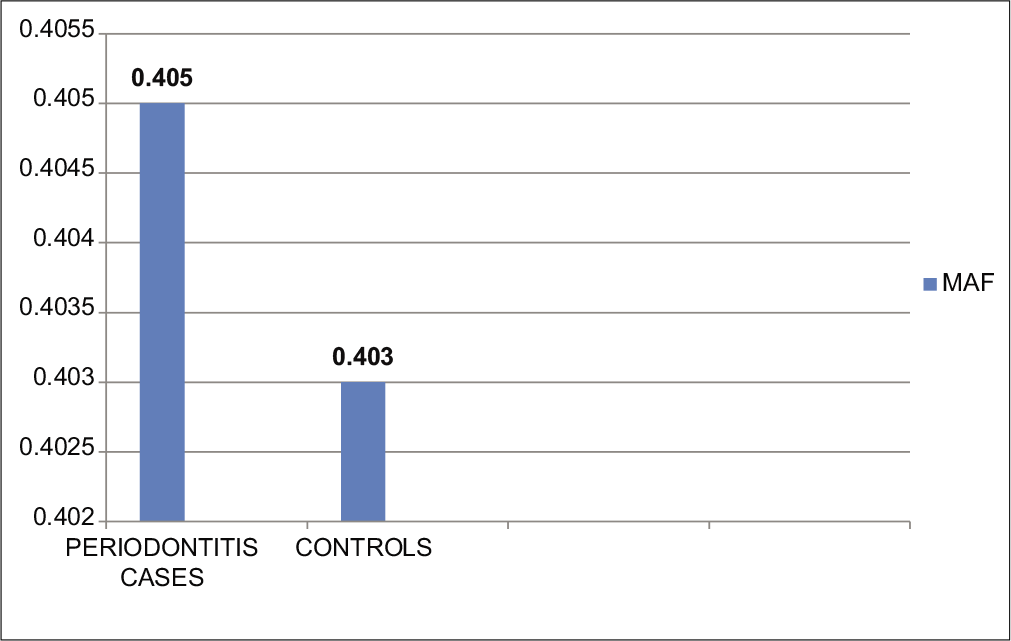
- Comparison of minor allele frequency between periodontitis cases and controls. MAF: Minor allele (g allele) frequency.
DISCUSSION
The genetic basis of the susceptibility to periodontal disease has been supported by several convincing evidence. However, findings of individual research have been inconclusive. Regional and racial differences are likely to be the reasons for the disparity in the results. Lopez et al., 2009, evaluated the significant association between IL-1β-511 CC genotype and periodontitis in a Chilean population.[12] Karasneh et al., 2011, demonstrated that the C allele and TC genotype of IL-1β+3954,-511 were increased in chronic and aggressive periodontitis in a Jordanian population.[13] Trevilatto et al., in 2011,[7] demonstrated difference in the genotype (P = 0.051) and allele (P = 0.018) frequencies between the healthy group and groups with periodontitis in the subgroup comprising the Afro-Americans and mulattos for the IL1β-511 locus. The frequency of T allele was higher in cases than controls and there was a higher prevalence of T/T homozygous genotype in cases than in controls. Similarly, Amirisetty et al. revealed a strong association of IL-1β-511 (rs16944) TT genotype with chronic periodontitis in North Indian population,[14] whereas our present study showed higher prevalence of IL-1β-511 (rs16944) AG genotype in a group of the Bengali population of West Bengal state. However, CC genotype has been the more frequently found genotype in periodontitis cases than in controls in a sample of Indian population of Karnataka state.[15] Wang et al., 2017, identified a strong association between the IL-1β C-511T (rs16944) polymorphism and periodontitis in Chinese population.[16] However, Nikolopoulos et al. in 2008 observed weak positive association for IL1β-511 genotypes in CP patients.[8] Zeng et al. in 2015 through their updated meta-analysis suggested a non-significant association between IL-1β C-511T polymorphism and CP.[17] The present study suggested no association between IL-1β-511 (rs16944) and periodontitis in our patient cohort. The frequency of IL-1β-511 (rs16944) allele 2 homozygous genotypes GG was found to be higher in the control group (9.7%) than in overall periodontitis cases (3.5%). The minor allele frequencies of both periodontitis cases and controls were 0.405 and 0.403, respectively (odds ratio = 1.008; 95% CI = 0.53–1.88; P = 0.9799). However, the present study has certain limitations. The sample size was small and Bengali subjects were selected based on their names rather than through genealogical study. Further well-designed study with larger sample size is necessary.
CONCLUSION
The present study revealed no association of single nucleotide polymorphism of IL-1β-511 (rs16944) with total periodontitis cases (both CP and AgP) in our patient cohort. The frequency of IL-1β-511 (rs16944) allele 2 homozygous genotypes (GG) was found to be higher in the control group (9.7%) in comparison to overall periodontitis cases (3.5%). Further longitudinal study is essential to validate the biologic basis for genetic susceptibility testing, to establish the polymorphism of IL-1β-511 (rs16944) as a genetic marker for susceptibility to periodontitis. However, the ideal method of elucidating any association of genetic polymorphism with periodontitis would be to start before the onset of the disease and following it up through the natural history. To accomplish this, the genotypes required have to be established first and then follow it up as the subject is exposed to various predisposing and risk factors.
Acknowledgments
We extend our heartfelt gratitude and acknowledgment to Dr. Raghunath Chatterjee (Associate Prof.), Ms. Aditi Chandra (CISR-JRF), and Mr. Shantanab Das (CISR-JRF), Human Genetics Unit, Biological Science Division, Indian Statistical Institute, Kolkata.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Microbial etiological agents of destructive periodontal diseases. Periodontol 2000. 1995;5:78-11.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiologic pattern of chronic and aggressive periodontitis. Periodontol 2000. 2010;53:28-44.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of periodontitis in the Indian population: A literature review. J Indian Soc Periodontol. 2011;15:29-34.
- [CrossRef] [PubMed] [Google Scholar]
- The interleukin-1 genotype as a severity factor in adult periodontal disease. J Clin Periodontol. 1997;24:72-7.
- [CrossRef] [PubMed] [Google Scholar]
- Association of IL1 gene polymorphisms with chronic periodontitis in Brazilians. Arch Oral Biol. 2011;56:54-62.
- [CrossRef] [PubMed] [Google Scholar]
- Cytokine gene polymorphisms in periodontal disease: A metaanalysis of 53 studies including 4178 cases and 4590 controls. J Clin Periodontol. 2008;35:754-67.
- [CrossRef] [PubMed] [Google Scholar]
- Periodontal disease in pregnancy. II. Correlation between oral hygiene and perio-dontalcondition. Actaodont Scand. 1964;22:112-35.
- [CrossRef] [PubMed] [Google Scholar]
- The simplified oral hygiene index. J Am Dent Assoc. 1964;68:7-13.
- [CrossRef] [PubMed] [Google Scholar]
- Periodontal disease in pregnancy. I. Prevalence and severity. Actaodont Scand. 1963;21:533-51.
- [CrossRef] [PubMed] [Google Scholar]
- Interleukin-1 gene cluster polymorphisms associated with periodontal disease in Type 2 diabetes. J Periodontol. 2009;80:1590-8.
- [CrossRef] [PubMed] [Google Scholar]
- Investigation of the interleukin-1 gene cluster polymorphisms in Jordanian patients with chronic and aggressive periodontitis. Arch Oral Biol. 2011;56:269-76.
- [CrossRef] [PubMed] [Google Scholar]
- Interleukin 1beta (+3954,-511 and-31) polymorphism in chronic periodontitis patients from North India. Acta Odontol Scand. 2014;2014:1-5.
- [CrossRef] [PubMed] [Google Scholar]
- Allele, genotype and composite genotype effects of IL-1A+4845 and IL-1B+3954 polymorphism for chronic periodontitis in an Indian population. Indian J Dent Res. 2011;22:612.
- [CrossRef] [PubMed] [Google Scholar]
- Association between the interleukin-1β C-511T polymorphism and periodontitis: A meta-analysis in the Chinese population. Genet Mol Res. 2017;16:1-9.
- [CrossRef] [PubMed] [Google Scholar]
- Meta-analysis of association between interleukin-1β C-511T polymorphism and chronic periodontitis susceptibility. J Pereiodontol. 2015;86:1-16.
- [CrossRef] [PubMed] [Google Scholar]







