Hypothesis: Relationship between Coronavirus Disease-19 and Periodontal status

*Corresponding author: Dr. Shruti Ligade, Department of Periodontology, Dr. D Y Patil Vidyapeeth, Pune, Maharashtra, India. drshrutiligade2530@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Ligade SS, Shah YS. Hypothesis: Relationship between Coronavirus Disease-19 and Periodontal status. J Global Oral Health 2021;4(1):48-55.
Abstract
Coronavirus disease 2019 (COVID-19) is a global pandemic affecting 185 countries and >18.8 million patients worldwide as of July 2020. COVID-19 is caused by severe acute respiratory syndrome coronavirus 2 (SARS-2) and majorly manifesting as a hypoxic condition in the affected patients. It has shown to multiply rapidly among patients showing other debilities, majorly hypertension, and diabetes mellitus. Periodontitis is defined as a multifactorial disease predominantly governed by microbial dysbiosis and having risk factors as the presence of systemic pathology, decline in immunity, and increased stress. Keeping these factors as a plausible threat for periodontitis, there can be a postulation made that periodontitis can act as a probable risk factor for COVID-19. Limited resources and novelty of the disease hold back any confirmation of the co-relation.
Keywords
Coronavirus disease 2019 and dentistry
Coronavirus and periodontitis
Hypothesis: Coronavirus and oral disease
Coronavirus disease 2019 and related diseases
INTRODUCTION
As the world got hit by “novel” coronavirus everyone stood together to race against time and help fight this pandemic. We all are trying to find ways to treat and prevent the disease and develop vaccines. In this scrambling fight against the disease, various findings have come forth which help us deal with the situation a little better. One such hypothesis is the presence of risk factors in an individual which predisposes them to the disease. In this article, we will hypothesize and explore the extent of the possibility of periodontal disease as one of the risk factors as well as the significance of periodontal investigation in COVID-19 patients.
EVOLUTION AND CLASSIFICATION
Coronaviruses are a large family of single positive-stranded, enveloped RNA viruses that can infect many animal species and humans. Human coronaviruses can be divided based on their pathogenicity. The coronavirus infestation manifesting as severe acute respiratory syndrome coronavirus (SARS) is called SARS-CoV. According to the World Health Organization (WHO), the first cluster of SARS cases occurred in China’s Guangdong province in November 2002. The WHO officially declared the SARS epidemic to be contained on July 5 of that year. Middle East respiratory syndrome (MERS) occurs as a result of infection with the coronavirus MERS-CoV, the first case of which was reported on September 20, 2012. MERS-CoV which is defined as a zoonotic virus transfers passage of infection from animals to humans, predominantly dromedary camels.
The coronavirus SARS-CoV-2 is the virulence factor that causes COVID-19; the virus has a close resemblance to SARSCoV. It falls under the beta CoVs category. The first cases of COVID-19 were reported in Wuhan, China, in December 2019 and accounted in India on January 30, 2020. The name COVID-19 was officially coined, by the WHO, on February 11. Exactly 1 month later, the organization declared a pandemic.[1]
EPIDEMIOLOGY
The initial COVID-19 cases were associated with travel history to Hubei Province, China; however, a growing number of cases due to person-to-person transmission have been reported both in and outside of China.[2] Up to 94% of COVID-19 cases were reported to originate from Hubei Province in December 2019. As of July 2020, the greatest number of new cases are now being reported in several countries like United States, Russia, South Africa and Brazil. India currently stands the 3rd worst hit nation on global standards which is believed to be predominantly through community transmission.
SARS and MERS have significantly higher case fatality rates than COVID-19. Yet COVID-19 is more infectious — the underlying SARS-CoV-2 virus spreads more easily among people, leading to greater case numbers.
Despite the lower case fatality rate, the overall number of deaths from COVID-19 far outweighs that from SARS or MERS.[3] COVID-19 appears to transmit more easily than SARS. One possible explanation is that the amount of virus, or viral load, appears to be highest in the nose and the throat of people with COVID-19 shortly after symptoms develop.[1]
VIRAL MANIFESTATIONS
The majority of infected people have no symptoms or just mild. The severity of disease and the mortality rate of COVID-19 cases increases with age and in people with pre-medical conditions such as heart, lung, kidney, or liver disease, diabetes, immunocompromising conditions, or severe obesity. Severe symptoms are characterized by dyspnea, hypoxia, and extensive lung involvement, as seen after radiological imaging. Severe cases are characterized by tachypnea (>30 breaths/min) and hypoxia (oxygen saturation SpO2 <90% on room air). This can progress to respiratory failure requiring mechanical ventilation, shock, and multiple organ failure.[4]
Periodontitis is a chronic inflammatory disease induced by opportunistic Gram-negative anaerobic bacteria at the tooth-supporting apparatus. Within the gingiva, the affected sulcus or periodontal pocket, the resident anaerobic bacteria interact with the host inflammatory reactions leading to lower oxygen or hypoxic environment. The biological relevance of hypoxia in periodontal/oral cellular development, epithelial barrier function, periodontal inflammation, and immunity has already been reviewed.[5] Respiratory pathogens have the potential for oral colonization, especially among institutionalized, elderly, and debilitated patients. The dysbiosis that occurs from the native periodontal ecological system to pathogenic microorganisms is well documented. It has also been recorded that microorganisms released from saliva can get aspirated into the respiratory tract causing or aggravating respiratory infections.[6] Since COVID-19 shows respiratory disorders as its major manifestations, this study postulates to be any possible periodontal manifestation by the viral infestation. Since COVID-19 shows respiratory disorders as its major manifestations, this study postulates any possible periodontal manifestation by the viral infestation.
HYPOXIA AND ORAL MICROBIOTA
Various environmental factors and host factors are involved in the harboring of microorganisms and microbial composition. Many indigenous microbiota are anaerobes and these microorganisms can be associated with oral infections and be the origin of distant infection. The biofilm present in the gingival crevice, and later in the periodontal pocket, is extremely diverse, with up to 100 cultivable species from a single pocket.[7] Moore and Moore presented large numbers of anaerobes increase in their overall proportions during disease progression and, conversely, aerobe and facultative species decrease.[8] The long-standing paradigm is that as periodontitis develops, the oral microbiota shifts from one consisting primarily of Gram-positive aerobes to one consisting primarily of Gram-negative anaerobes. The development of oral dysbiosis is likely to occur over an extended period of time, gradually changing the symbiotic host-microbe relationship to a pathogenic one. Thus, this change of microbiota from the “orange complex,” which consists of Gram-negative, anaerobic species such as Prevotella intermedia and Fusobacterium nucleatum, to the late colonizers “red complex,” namely, Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola shows the shift from periodontal health to disease following the alterations in oxygen availability, redox action, and shift in pH (potential for hydrogen). The shift from a symbiotic microflora to a dysbiotic, pathogenic community triggers the potent host inflammatory response which contributes to the tissue destruction and alveolar bone loss characteristic of periodontitis.[9]
HYPOXIA AND PERIODONTAL APPARATUS
When an animal acquires oxygen through its breathing apparatus, the oxygen passes under a reducing partial oxygen pressure (pO2) gradient from the source through circulation to different organs and then tissues and cells to the colonization by subgingival biofilm, oxygen is persistently consumed to various extents by the facultative anaerobic microbes within the periodontal sulcus (2.33–8.40 kPa).[10] Inside a periodontal pocket, the inflammation induced by the native anaerobic bacteria leads to even lower oxygen tension. At the tissue level, the availability of oxygen is dependent on the distance from the oxygen-supplying blood vessels which are estimated to be 100–200 μm. However, a pO2 of almost zero has been recorded in tissues 100 μm away from the nourishing blood vessels.[11] Cellular hypoxia, or a lower than “normal” concentration of O2 in cells, occurs commonly and could induce significant changes, immediate or delayed, on cellular processes, including cell growth and apoptosis, cell proliferation and survival, pH regulation and energy metabolism, cell migration, matrix and barrier function, angiogenesis, and vasomotor regulation.[12-14]
Therefore, a cellular/tissue oxygen-sensing mechanism is needed to assist tissue adaptation to variations in oxygen availability which is majorly mediated through a key cellular transcription factor named the hypoxia-inducible factor (HIF). Hypoxia, in the compensatory mechanism, induces the expression of a number of angiogenic factors to improve the blood supply in needed areas, including inflamed periodontium. These include vascular endothelial growth factor, platelet-derived growth factor, and angioprotein-1 and angioprotein-2.[15] HIF activation promotes a metabolic switch to reduce oxygen consumption by shifting energy metabolism from aerobic respiration to glycolysis which is essential for the host’s defense because such HIF-1 alpha–regulated glycolytic metabolism is required in B cell development and T cell metabolism.[16,17]
At sites where a chronic inflammatory reaction occurs, oxygen consumption is elevated, and blood perfusion is stimulated, but the actual local microcirculation could be compromised. This, in turn, increases the anaerobic microbial load. Certain periodontopathogens, as P. gingivalis LPS under hypoxia, increase PDL fibroblasts’ oxidative stress and induce a reduction of catalase. This indicates that there is a collapse of the protective mechanism for increasing reactive oxygen species and the progression of inflammatory oral diseases.[18] The symbiotic relationship between gingival microbes could be a potential reason for the high viral proliferation in the gingival sulcus. It is presumed that the virus modulates the local microbial environment resulting in the retention of bacterial colonies. This, in turn, aids in triggering the reactivation of viruses.[19]
A recent study showed that a conducive symbiotic local microbial environment and the presence of angiotensin-converting enzyme (ACE2) inhibitors can postulate that gingival sulcus is a potential ecological niche for SARS-CoV-2.[20]
Further studies must be carried out to confirm the hypothesis, the presence of SARS-CoV-2 in the gingival crevicular fluid, and salivary samples of COVID-19 patients both during the disease progression and post-recovery. SARS-CoV-2 has been detected in the saliva of confirmed patients with COVID-19, even up to the 11th day after hospitalization, in one of the cases.[21] This shows the possibility that patients in convalescence might be carriers of the SARS-CoV-2 in the gingival crevicular fluid.
In human monocytes, lipopolysaccharides (LPS) and hypoxia synergistically activate HIF-1 through p44/42 mitogen-activated protein kinases and nuclear factor kappa B cells (NF-κB); however, repetitive exposure to LPS could induce tolerance to bacterial endotoxins and, hence, impair corresponding HIF-1α induction, which reduces the ability of monocytic cells to survive and function under low oxygen.[22,23]
CO-RELATION WITH CYTOKINES
As the novel coronavirus is taking the world by a storm, there is another kind of storm that is brewing inside, a cytokine storm.
Hypercytokinemia or cytokine storm is associated with diseases such as SARS, MERS, and influenza. Recent evidence shows linkages of cytokine storm with COVID-19 and is associated with the severity of the disease.[24] A cytokine storm entails elevated serum levels of interleukins as IL-1 beta, IL-7, IL-10, IL-17, IL-2, IL-8, IL-9, granulocyte-macrophage colony-stimulating factor, interferon-gamma, tumor necrosis factor-alpha, MIP1A, MIP1B, MCP1, and IP10.[25] Th17 type of inflammatory response is involved in the manifestation of the cytokine storm and adverse outcomes pertaining to pulmonary edema and tissue damage in lung infections, including that caused by COVID-19. Many researchers are proposing for the COVID-19 affected patients with anti-cytokine storm therapy.[26] Although periodontal disease is caused by a bacterial infection, a lot depends on the host’s response to the disease. The severity with which the disease would manifest depends on that response. The event between bacterial stimulation and tissue destruction is an inflammatory response. It has been proven that cytokines IL-17 are present in patients with periodontal diseases and are responsible for periodontal destruction.[27]
It has also been proven that the treatment of periodontal diseases leads to a decrease in inflammation due to a decrease in IL-17 levels.[28]
Risk is the probability that an individual will develop a given disease or exposure or change in health status in a given period. This points to a possibility that periodontal disease might be a risk factor in the increased severity of COVID-19 manifestation. It is also a possibility that the treatment of periodontal disease will help reduce the severity of COVID-19 in affected patients.[29]
HIF AND BONE HOMEOSTASIS
HIF appears to play important functional roles in bone homeostasis. The regulatory system seems complex because HIF is known to stimulate both bone resorption and regeneration, the two essential biological processes in bone homeostasis/repair.
It is reported that a lack of oxygen in periodontal tissues may contribute to alveolar bone resorption and, in theory, accelerated periodontitis. Chromatin immunoprecipitation showed that HIF-1α binds to the receptor activator of the NF-κB ligand promoter region, and mutations of the putative HIF-1α binding site prevented hypoxia-induced receptor activator of nuclear factor-kappa-B ligand (RANKL) transcriptional promotion. This suggests that HIF-1α mediates hypoxia-induced upregulation of RANKL expression and enhances osteoclastogenesis. Furthermore, it was reported that hypoxia triggered the differentiation of peripheral mononuclear blood cells into functional osteoclasts in a HIF-dependent manner. A recent animal study reported new bone and vessel formation induced by the over-expression of HIF-1α through adenovirus, leading to enhanced alveolar bone defect regeneration.[30] Cementoblastic differentiation of human dental stem cells, a key cellular mechanism concerning periodontal regeneration, was reported to be stimulated by hypoxia in a HIF-1-dependent manner.[31]
The mechanisms underlying the role of HIF-1 and periodontal defense/pathogenesis, however, remain elusive. Over- or under-activation of the immune system with or without the corresponding dysregulation of HIF-1 biology in tissues as well as alveolar bone, however, could give rise to periodontal tissue damages.[32]
NECROTIZING ULCERATIVE PERIODONTITIS (NUP)
The role of immune dysfunction is exemplified by the aggressive nature of necrotic forms of periodontal disease which are seen in patients with human immune virus (HIV) infection, malnutrition, stress, or culminative of all these factors. All of these have proven to negatively impact the host defenses. The 1992 edition of the Glossary of Periodontal Terms defines NUP as “severe and rapidly progressive disease that has distinctive erythema of the free gingiva, attached gingiva, and alveolar mucosa; extensive soft tissue necrosis and severe loss of periodontal attachment. Although the etiology of NUP is not specified, there are various predisposing factors such as stress, nutritional deficiencies, and immune system dysfunctions, especially HIV infection that seems to play a major role in the pathogenesis of NUP. The existence of immune dysfunction may predispose patients to NUP, especially when associated with an infection of microorganisms as Treponema and Spirochetes species. The role of Gram-negative anaerobic Spirochetes in disease pathogenesis is associated with levels of oxygen in periodontal tissue. Loesche et al. studied the role of oxygen tension and composition of microbial flora in periodontal pockets and found that microbial load was significantly elevated in the pockets with low PO2. Deep pockets were found to have both a lower P02 relative to moderate pockets and to contain higher proportions of Spirochetes. Thus, the oxygen sensitivity of the plaque organism coincided with its proportional distribution in pockets of various P02 and depths. P02 readings in periodontal pockets indicated that there is a spectrum of P02 values which seems to define, in a general way, the microbiological composition of the pocket. Thus, in pockets where the P02 values are low, all sizes of Spirochetes increase proportionally.[33] Taking into consideration all these factors, it can be directly postulated that finding of NUP in COVID-19 affected patients is highly possible.
Another interesting association was recorded between a reduced oxygen environment and the occurrence of Candida albicans which is opportunistic pathogenic yeast. Rosa et al. found that secretory aspartyl proteases secretion consistently increased in cultures of C. albicans strains when strains recovered from periodontal pockets were grown under anaerobic conditions. This suggests that oxygen concentration in the atmosphere surrounding cells influences the virulence attributes of C. albicans.[34]
ASSOCIATION OF CO-MORBIDITIES
This study strives to conceptualize the plausible association of periodontitis and the manifestations in COVID-19. The possible physiology is mapped in [Figure 1].
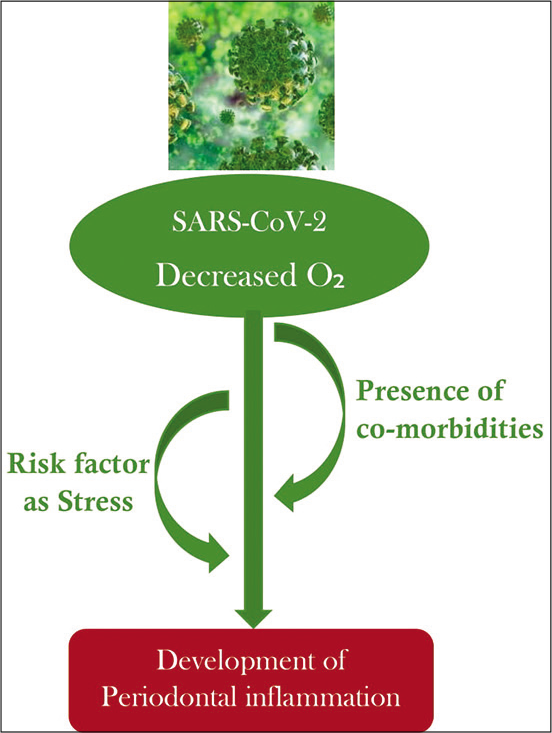
- Flowchart signaling the possible progress of COVID-19 infestation in the periodontal apparatus.
Older people and people with pre-existing medical conditions (such as diabetes, heart diseases, and asthma) appear to be more vulnerable and undergo serious symptoms of the COVID-19 virus. It is found that COVID-19 patients with underlying health conditions are more likely than others to get severely ill from viral manifestation. Other medical conditions such as obesity, diabetes, and serious heart that place them at higher risk of severe illness from COVID-19.[35]
PERIODONTITIS AS A RISK FACTOR OF CARDIOVASCULAR DISEASE (CVD)
Patients with cardiac risk factors and established CVDs seem to have an increased vulnerability to develop COVID-19 and tend to have increased severity of the disease with a worse clinical outcome.[36] Multiple mechanisms have been suggested for cardiac damage based on studies on previous SARS and MERS epidemic and the currently outgoing COVID-19.
COVID-19 shows high levels of cytokines surge, which results in injury to multiple organs, including cardiac myocytes.[37] Studies have shown elevated levels of pro-inflammatory cytokines in patients with severe COVID-19 disease. ACE receptors expressed in both Type 1 and Type 2 pneumocytes are also found in other cells such as endothelial cells. ACE-2 is an inverse regulator of the Renin-angiotensin system.
The oxygen supply-demand in myocardial cells, if it results in a mismatch, leads to increase cardiometabolic demand associated with systemic infection and hypoxia caused by pneumonia or acute respiratory distress syndrome. This leads to increased demand, and the inadequate supply leads to myocardial damage.[37] Willershausen et al. demonstrated that there was a strict association between chronic dental infection and acute myocardial infarction.[38] Further, Jansson et al. suggested that oral disease could be used as a risk indicator of death due to CVD, especially when this was combined with other well-established risk factors such as smoking.[39]
Periodontitis is a multifactorial, chronic inflammatory disorder, and the augmented concentration of bacterial surface molecules, such as LPS, stimulates the production of inflammatory mediators and cytokines that, in turn, promote the release of the matrix metalloproteinases. These tissue-derived enzymes then participate in extracellular matrix remodeling and bone destruction. Importantly, recent studies have clearly proven that these deleterious effects are not only restricted to the oral cavity but can affect the overall health of an individual. Indeed, periodontal pathogens can destroy the epithelium of the periodontal pocket, thus allowing the entry of noxious endotoxins and exotoxins into the bloodstream. This process leads to bacterial dissemination and systemic infection, with a consequent rise in inflammatory response like in acute bacterial endocarditis. For these reasons, in the last decades, periodontitis has been associated with the onset of systemic disorders, including CVDs and diabetes. Furthermore, it has been previously demonstrated that individuals with periodontitis have a sensible increased risk of developing CVDs, including myocardial infarction, heart failure, peripheral artery disease, atherosclerosis, and stroke.
Endothelial dysfunction is an independent predictor of cardiovascular events and precedes the development of atherosclerosis and other CVDs. This pathological process is usually caused by the reduced bioavailability of endogenous molecules such as nitric oxide.[40] In this regard, a recent meta-analysis has demonstrated the beneficial effects of periodontal therapy on endothelial function. Orlandi et al. observed that periodontal disease was associated with greater carotid intima-media thickness and with impaired flow-mediated dilation (FMD). This indicates the presence of atherosclerosis and endothelial dysfunction, respectively. Nevertheless, in patients that have undergone intensive periodontal treatments, a substantial improvement in the periodontal condition was seen along with an increased FMD.[41]
DIABETES AND PERIODONTAL DISEASE: A TWO-WAY RELATIONSHIP
When people with diabetes develop a viral infection, it can be harder to treat due to fluctuations in blood glucose levels and possibly the presence of diabetic complications. There appear to be two reasons for this; first, the immune system is compromised, making it harder to fight the virus and likely need a longer recovery period. Second, the virus may thrive in an environment of elevated blood glucose levels.[42]
Diabetes mellitus (DM) is a clinical syndrome, characterized by hyperglycemia, caused by inherited and/or acquired deficiency in insulin production and/or action. The interrelationship in periodontitis and DM has been found by Löe in 1993 and periodontal disease is considered as the 6th complication of diabetes.[43] Of note, several reports have suggested that DM participates in altering the subgingival bacterial community through substrate-related alterations offering a microenvironment favorable for the pathogenic growth.[44] Quintero et al. have demonstrated that periodontal therapy can also reduce HbA1c levels in subjects with DM.[45]
Finally, oxidative stress appears to be another major link between DM and periodontitis since it can activate pro-inflammatory pathways common to these pathologies. In this regard, Patil et al. have observed that DM, in patients with periodontitis, is associated with higher levels of plasma biomarkers of oxidative stress that may activate systemic pro-inflammatory pathways.[46]
A meta-analysis of six published studies from China, including 1527 patients with COVID-19, reported. 9.7%, 16.4%, and 17.1% prevalence of diabetic mellitus, cardiocerebrovascular disease, and hypertension, respectively. The overall case fatality rate (CFR) was 2.3%.[47]
A recent 2020 study by Guan et al. showed that the presence of any comorbidity yielded poorer clinical outcomes than those without. A greater number of comorbidities also correlated with poorer clinical outcomes, namely, hypertension (16.9%) followed by diabetes (8.2%).[48]
Based on this premise, it is of paramount importance to inform health professionals about the consequence of diseases affecting the oral cavity in that these are potentially associated with a range of pathologic conditions.
To conclude, it can be stated that several pre-existing conditions as mentioned above can prove as a risk factor for periodontitis. There’s increasing evidence of the same for COVID-19 as well. Sharing the multiple risk factors could lead to a hypothesis that pre-existing periodontitis could lead to an increased severity of COVID-19.
CO-RELATION WITH STRESS
The association of stress with the emergence of periodontal disease has been established. Stress, directly and indirectly, affects immunity and thus bodily resistance is breached. In this recent pandemic, there has been an equal congruency found in COVID-19 manifestation and its effect on mental stress and well-being.
Manhold et al. tested the hypothesis that in long or continued emotions, a constant constriction of the blood vessels would produce a lack of oxygen and nutrient materials for the periodontal tissues. They found a lower ability of the tissues of rats under stress to utilize oxygen.[49]
Psychiatric disturbances such as anxiety, disturbance, and impact of negative life events may lead to activation of hypothalamic-pituitary-adrenal axis. This results in elevation of serum and urine cortisol levels which is associated with depression of lymphocytes and neutrophil function. This can independently predispose to NUP.[50] A recent meta-analysis studied in May 2020 indicated that in the post-illness stage, the point prevalence of post-traumatic stress disorder was 32•2%, that of depression was 14•9% and that of anxiety disorders was 14•8%. The etiology of the psychiatric consequences in a COVID-19 positive patient is multifactorial. The reasons might include the direct effects of viral infection, cerebrovascular disease (including in the context of a procoagulant state), the degree of physiological compromise (e.g., hypoxia), the immunological response, medical interventions, social isolation, the psychological impact of a novel severe and potentially fatal illness, concerns about infecting others, and stigma. The immune response in SARS-CoV-2 infection is of interest and there might be a hyperinflammatory state.[51] Taylor et al. anticipated that when this pandemic passes, significant mental health needs will emerge in public. Neural imbalances as anxiety, depression, and traumatic reactions were observed especially due to COVID-19 danger and contamination fears, economic crises, and xenophobia.[52] Taking into consideration this close association, it can be steadily stated that periodontal status in COVID-19 patients should be checked and possible manifestations of periodontitis in COVID-19 patients with stress should be anticipated.
CONCLUSION
COVID-19, caused by SARS-CoV-2, is a global pandemic evolving in real time. While most people with COVID-19 develop only mild or uncomplicated illness, approximately 14% develop a severe disease that requires hospitalization and oxygen support, and 5% require admission to an intensive care unit under the treatment of ventilators. Hypoxia affects a larger population of patients and its consequences on periodontal status have been discussed in the study. The initial or advanced symptoms of COVID-19 are yet aggravated on the presence of comorbidities. The study presents the data which confirmed that various comorbidities were found common in patients with COVID-19 and these patients pose a higher risk of morbidity and mortality. As the world struggles with the virus itself, also does it parallelly fights stress related to the disease. Stress ambiguously has shown adverse effects on periodontal conditions. Thus, cumulatively it can be postulated that periodontitis could pose a risk factor for COVID-19. Similarly, manifestations of periodontal disease should be inspected in COVID-19 patients to realize the combined impact of various etiologies.
Limitations
The study reviews the initial case reports and research articles which got documented as the intensity of COVID-19 grew from countable cases to become a pandemic virus. Further studies are required to determine the same. If clinical research evaluating the actual correlation of the periodontal disease status in COVID-19 patients is done, that will prove to be a flag bearer for concluding studies.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-adetail/q-a-coronaviruses [Last accessed on 2020 Aug 30]
- Clinical characteristics of 138 hospitalized patients with 2019 novel Coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061-9.
- [CrossRef] [PubMed] [Google Scholar]
- SARS-CoV-2 and COVID-19: The most important research questions. Cell Biosci. 2020;10:40.
- [CrossRef] [PubMed] [Google Scholar]
- Available from: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html [Last accessed on 2020 Aug 30]
- Role of the Hypoxia-Inducible Factor in Periodontal Inflammation. London: Intech Open; 2017
- [CrossRef] [Google Scholar]
- Respiratory disease and the role of oral bacteria. J Oral Microbiol. 2010;2
- [CrossRef] [PubMed] [Google Scholar]
- Periodontal infection as a possible risk factor for preterm low birth weight. J Periodontol. 1996;67:1103-13.
- [CrossRef] [Google Scholar]
- The bacteria of periodontal diseases. Periodontol 2000. 1994;5:66-77.
- [CrossRef] [PubMed] [Google Scholar]
- Microbial ecology of dental plaque and its significance in health and disease. Adv Dent Res. 1994;8:263-71.
- [CrossRef] [PubMed] [Google Scholar]
- Tissue oxygen tension and brain sensitivity to hypoxia. Respir Physiol. 2001;128:263-76.
- [CrossRef] [Google Scholar]
- Oxygen sensing and signaling: Impact on the regulation of physiologically important genes. Respir Physiol. 1999;115:239-47.
- [CrossRef] [Google Scholar]
- HIF-1α-PDK1 axis-induced active glycolysis plays an essential role in macrophage migratory capacity. Nat Commun. 2016;7:11635.
- [CrossRef] [PubMed] [Google Scholar]
- Hypoxia-induced microRNA-301b regulates apoptosis by targeting Bim in lung cancer. Cell Prolif. 2016;49:476-83.
- [CrossRef] [PubMed] [Google Scholar]
- Overcoming hypoxia-mediated tumor progression: Combinatorial approaches targeting pH regulation, angiogenesis and immune dysfunction. Front Cell Dev Biol. 2016;4:27.
- [CrossRef] [PubMed] [Google Scholar]
- Expression of hypoxia-inducible factor-1α in human periodontal tissue. J Periodontol. 2011;82:136-41.
- [CrossRef] [PubMed] [Google Scholar]
- Differentiation stage-specific requirement in hypoxia-inducible factor-1alpha-regulated glycolytic pathway during murine B cell development in bone marrow. J Immunol. 2010;184:154-63.
- [CrossRef] [PubMed] [Google Scholar]
- Hypoxia-inducible factors regulate T cell metabolism and function. Mol Immunol. 2015;68:527-35.
- [CrossRef] [PubMed] [Google Scholar]
- LPS from P. gingivalis and hypoxia increases oxidative stress in periodontal ligament fibroblasts and contributes to periodontitis. Mediators Inflamm. 2014;2014:986264.
- [CrossRef] [PubMed] [Google Scholar]
- Herpesvirus-bacteria synergistic interaction in periodontitis. Periodontol 2000. 2020;82:42-64.
- [CrossRef] [PubMed] [Google Scholar]
- Is the gingival sulcus a potential niche for SARS-Corona virus-2? Med Hypotheses. 2020;143:109892.
- [CrossRef] [PubMed] [Google Scholar]
- Consistent detection of 2019 novel Coronavirus in saliva. Clin Infect Dis. 2020;71:841-3.
- [CrossRef] [PubMed] [Google Scholar]
- Hypoxia-inducible factor (HIF) 1alpha accumulation and HIF target gene expression are impaired after induction of endotoxin tolerance. J Immunol. 2009;182:6470-6.
- [CrossRef] [PubMed] [Google Scholar]
- Bacterial lipopolysaccharide induces HIF-1 activation in human monocytes via p44/42 MAPK and NF-kappa B. Biochem J. 2006;396:517-27.
- [CrossRef] [PubMed] [Google Scholar]
- The serum profile of hypercytokinemia factors identified in H7N9-infected patients can predict fatal outcomes. Sci Rep. 2015;5:10942.
- [CrossRef] [PubMed] [Google Scholar]
- TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor fedratinib. J Microbiol Immunol Infect. 2020;53:368-70.
- [CrossRef] [PubMed] [Google Scholar]
- A promising anti-cytokine-storm targeted therapy for COVID-19: The artificial-liver blood-purification system. Engineering (Beijing) 2020
- [CrossRef] [PubMed] [Google Scholar]
- Cytokines that promote periodontal tissue destruction. J Periodontol. 2008;79(Suppl 8):1585-91.
- [CrossRef] [PubMed] [Google Scholar]
- The presence, function and regulation of IL-17 and Th17 cells in periodontitis. J Clin Periodontol. 2014;41:541-9.
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19 and periodontitis: The cytokine connection. Med Hypotheses. 2020;144:109908.
- [CrossRef] [PubMed] [Google Scholar]
- Application of HIF-1α by gene therapy enhances angiogenesis and osteogenesis in alveolar bone defect regeneration. J Gene Med. 2016;18:57-64.
- [CrossRef] [PubMed] [Google Scholar]
- Hypoxia promotes CEMP1 expression and induces cementoblastic differentiation of human dental stem cells in an HIF-1-dependent manner. Tissue Eng Part A. 2014;20:410-23.
- [CrossRef] [PubMed] [Google Scholar]
- The role of hypoxia in orthodontic tooth movement. Int J Dent. 2013;2013:841840.
- [CrossRef] [PubMed] [Google Scholar]
- Relationship between oxygen tension and subgingival bacterial flora in untreated human periodontal pockets. Infect Immun. 1983;42:659-67.
- [CrossRef] [PubMed] [Google Scholar]
- Phenotypic evaluation of the effect of anaerobiosis on some virulence attributes of Candida albicans. J Med Microbiol. 2008;57:1277-81.
- [CrossRef] [PubMed] [Google Scholar]
- Available from: https://www.cdc.gov/coronavirus/2019-ncov/faq.html [Last accessed on 2020 Aug 30]
- Clinical features of patients infected with 2019 novel Coronavirus in Wuhan, China. Lancet. 2020;395:497-506.
- [CrossRef] [Google Scholar]
- Cardiac Manifestations of Coronavirus (COVID-19) Treasure Island, FL: Stat Pearls Publishing; 2020.
- [Google Scholar]
- Association between chronic dental infection and acute myocardial infarction. J Endod. 2009;35:626-30.
- [CrossRef] [PubMed] [Google Scholar]
- Relationship between oral health and mortality in cardiovascular diseases. J Clin Periodontol. 2001;28:762-8.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative pharmacology of endothelium-derived relaxing factor, nitric oxide and prostacyclin in platelets. Br J Pharmacol. 1987;92:181-7.
- [CrossRef] [PubMed] [Google Scholar]
- Association between periodontal disease and its treatment, flow-mediated dilatation and carotid intima-media thickness: A systematic review and meta-analysis. Atherosclerosis. 2014;236:39-46.
- [CrossRef] [PubMed] [Google Scholar]
- Available from: https://www.diabetesvoice.org/en/news/covid-19-and-diabetes [Last accessed on 2020 Aug 30]
- Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care. 1993;16:329-34.
- [CrossRef] [Google Scholar]
- Effects of non-surgical periodontal treatment on clinical response, serum inflammatory parameters, and metabolic control in patients with type 2 diabetes: A randomized study. J Periodontol. 2012;83:435-43.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of two periodontal treatment modalities in patients with uncontrolled type 2 diabetes mellitus: A randomized clinical trial. J Clin Periodontol. 2018;45:1098-106.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic periodontitis in type 2 diabetes mellitus: Oxidative stress as a common factor in periodontal tissue injury. J Clin Diagn Res. 2016;10:BC12-6.
- [CrossRef] [PubMed] [Google Scholar]
- Emerging Coronaviruses: Genome structure, replication, and pathogenesis. J Med Virol. 2020;92:418-23.
- [CrossRef] [PubMed] [Google Scholar]
- Comorbidity and its impact on 1590 patients with COVID-19 in China: A nationwide analysis. Eur Respir J. 2020;55:2000547.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of social stress on oral and other bodily tissues. II. Results offering substance to a hypothesis for the mechanism of formation of periodontal pathology. J Periodontol. 1971;42:109-11.
- [CrossRef] [PubMed] [Google Scholar]
- Psychiatric, psychosocial, and endocrine correlates of acute necrotizing ulcerative gingivitis (trench mouth): A preliminary report. Psychiatr Med. 1983;1:215-25.
- [Google Scholar]
- Psychiatric and neuropsychiatric presentations associated with severe Coronavirus infections: A systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7:611-27.
- [CrossRef] [Google Scholar]
- Development and initial validation of the COVID stress scales. J Anxiety Disord. 2020;72:102232.
- [CrossRef] [PubMed] [Google Scholar]






