Nanoparticles in caries prevention: A review

*Corresponding author: Angambakkam Rajasekaran PradeepKumar, Department of Conservative Dentistry and Endodontics, Thai Moogambigai Dental College and Hospital, Dr. MGR Educational and Research Institute (Deemed to be University), Chennai, Tamil Nadu, India. arpradeependo@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Tejaswi B, SreeVidya G, Sivapriya E, Archana D, PradeepKumar AR. Nanoparticles in caries prevention: A review. J Global Oral Health 2021;4(1):56-66.
Abstract
Nanotechnology is the branch of engineering that uses molecular machines with precise structures that are less than or equal to 0.1 μm in size. The word nano denotes 10 to the power of minus nine or 1 billionth. Treatment options for dental caries have been extensively studied; among them, the role of nanoparticles is of recent interest. Nanoparticles have shown promising results in the field of caries prevention because of their unique physical, mechanical, and biological characteristics. Nanosized systems have distinctive properties due to their increased surface-to-volume ratio and increased bioavailability toward cells and tissues. Furthermore, improved surface area results in better mechanical interlocking of nanoparticles to the resin matrix. They prevent dental caries by antimicrobial, remineralizing, and anti-inflammatory mechanisms. Although many nanoparticles have been studied for their role in caries prevention, only a few materials which were extensively studied are included in this review.
Keywords
Caries prevention
Gold nanoparticles
Hydroxyapatite
Nanoparticles
Silver nanoparticles
INTRODUCTION
Dental caries is a multifactorial disease process caused by a microbial imbalance in the oral biofilm, provoked by frequent exposure to fermentable carbohydrates, resulting in the demineralization of dental hard tissues.[1,2] The primary etiological agents involved in the initiation of caries are Streptococcus mutans, Actinomyces spp., and non-S. mutans streptococci. Other species that play a crucial role in caries production are species of Veillonella, Lactobacillus, Bifidobacterium, Propionibacterium, low-pH non-S. mutans streptococci, Actinomyces, and Atopobium.[3]
Treatment for dental caries involves both conservative and preventive approaches which aim for specific person-to-person risk assessment by early detection of the disease and efforts are made to reverse or arrest dental caries, preserving tooth structure.[2,4] A large number of patients are still affected by caries despite the efforts and advancements in caries management. The ideal goal of any intervention/treatment would be the prevention of tooth decay. Changing the local conditions at the sites at risk proves a challenge in caries prevention.[5] Calcium and phosphate ions can reduce tooth demineralization, thus preventing dental caries. The salivary concentration of these ions will determine whether remineralization or demineralization will occur.[6]
Fluoride is the most commonly used remineralizing agent in the prevention of dental caries in the early stages.[6] Fluoride can react with hydroxyapatite forming fluorapatite or fluoridated hydroxyapatite.[6,7] These remineralizing agents are supplied either in liquid or semisolid forms, which can be easily administered and have good patient acceptability. The main disadvantage/ hurdle is poor retention in the oral environment, resulting in suboptimal therapeutic concentration and outcome.[8] The most common delivery systems for agents against dental caries are toothpaste, gels, tablets, and mouth rinses.[9]
Recent literature states that nanotechnology can aid in the prevention and management of dental caries by controlling plaque and helping in remineralization of initial caries.[10,11] This branch of engineering uses molecular machines with precise structures that are less than or equal to 0.1 μm in size. The word nano denotes 10–9 or 1 billionth. A nanoparticle is almost 1000 times smaller compared to micro and it measures 1/80,000 of the diameter of a human hair.[12,13] Nanoparticle-based aqueous suspensions are incorporated in a gel or paste form and used for oral applications.[14] Nanoparticles are preferred in biology and material science because of their unique properties such as uniformity, conductance, and specialized optical properties.[15]
Antimicrobial nanoparticles could inhibit bacterial growth and thus dental caries.[16,17] Nanoparticles that penetrate biofilms (plaque) and damage the extracellular polysaccharide matrix can enhance antibacterial efficacy and reduce the initiation of drug or antimicrobial resistance.[18]
Nanotechnology helps in treating dental caries by two important approaches. The first approach is the remineralization process, which uses nano-materials with fluoride and calcium releasing ability, namely, calcium phosphate, calcium fluoride, hydroxyapatite, and fluorohydroxyapatite. The second approach involves the administration of antibacterial nanoparticles such as silver, quaternary ammonium polyethylene amine, and zinc oxide.[19,20] Better outcomes are achieved by the combination of these two approaches. This review will give an insight into applications of nanotechnology in the prevention of early carious lesions and its role in remineralization.
PROPERTIES AND CLASSIFICATION OF NANOPARTICLES
General properties
Nanosized systems have distinctive properties due to their increased surface-to-volume ratio and increased bioavailability toward cells and tissues.[21,22] Improved surface area results in the better mechanical interlocking of nanoparticles to the resin matrix.[23] Superior mechanical properties are achieved by the addition of inorganic ceramic nanoparticles which are brittle and hard.[4] There is improved resistance to crack propagation and higher fatigue strength due to the reduction in areas of stress concentration.[24] Optical properties such as surface finish and translucency are improved when nanosized fillers are used in restorative materials.[25] Furthermore, there is better control of biodegradability and biodegradation rates in comparison to conventional composite materials.[26,27] The nanoparticles are classified as antimicrobial, remineralizing, and anti-inflammatory agents [Figure 1].
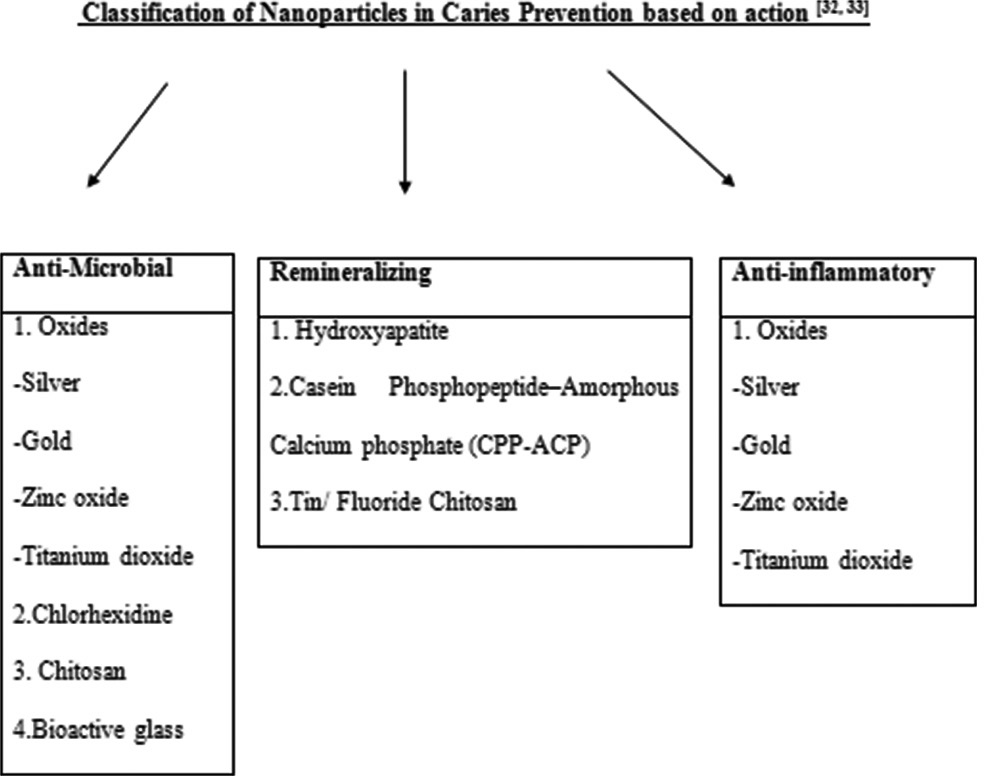
- Classification of nanoparticles in caries prevention based on action.
Anti-microbial and anti-inflammatory properties
The main etiological factor of dental caries is the presence of pathogenic bacteria that are organized within an extracellular matrix forming a biofilm.[28] The bacteria in biofilm are more resistant to antimicrobial treatment than planktonic organisms.[29,30] The nanoparticles have more effective antibacterial activity as the dimensions are reduced to the nanometer, resulting in an increased surface-to-volume ratio that allows them to interact and penetrate bacteria effectively.[31] Nanoparticles in caries prevention are classified based on their mechanism of action as mentioned in [Figure 1].[32]
SILVER NANOPARTICLES
Silver nanoparticles (Ag NPs) have been used for caries prevention in several studies.[33-44] These studies utilized silver nanoparticles in the form of silver nanocomposites, dentifrices, coated orthodontic brackets, nanosilver fluoride solutions, sealants, and glass ionomer cement with Ag NPs. In vitro research using Ag NPs and silver nanocomposites was done to treat and prevent secondary caries.[35,36] Ag NPs were also incorporated into the resin of orthodontic materials (adhesives, elastomeric ligatures, and removable retainers) for caries prevention.[37,38] Nanosilver fluoride solution was effective in remineralizing early enamel caries and arresting dentinal caries.[39-43] Clinical studies have proved that Ag NPs on orthodontic brackets can be used to prevent enamel caries[37] and that dental sealant with Ag NPs can be better than a traditional sealant in the prevention of enamel caries in first permanent molars.[44]
Antimicrobial activity
In vitro experiments
In vitro studies have shown that Ag NPs have an antimicrobial effect against Gram-positive bacteria such as Bacillus, Enterococcus, Listeria, Staphylococcus, Streptococcus, and Gram-negative bacteria such as Acinetobacter, Escherichia, Pseudomonas, and Salmonella.[45-48] The size, morphology, and concentration of nanoparticles play an important role in determining the antibacterial activity of Ag NPs. With the decrease in size of Ag NPs, the stability and biocompatibility increases. The higher surface-area-to-volume ratio of the smaller nanoparticles allows them to penetrate biological surfaces more readily.[49-51] Ag NPs smaller than 30 nm showed strong antimicrobial activity against Staphylococcus aureus and Klebsiella pneumoniae. Ag NPs with sizes ranging from 5 to 20 nm have strong antimicrobial activity against S. aureus.[52] Thus, small Ag NPs are more toxic against bacteria than large particles, and this further increased when the nanoparticles were oxidized.[53]
Silver ions are also released from materials that penetrate through microbial membranes and disrupt deoxyribonucleic acid replication and protein synthesis.[54] These ions can also deactivate respiratory enzymes and ultimately cause cell lysis. Ag NPs can accumulate on the pits of the cell wall and cause membrane denaturation.[55]
Ahmed et al. evaluated the action of toothpaste with and without Ag NPs against S. mutans[34] and reported that Ag NPs had antimicrobial activity against S. mutans. The mean diameter of the zone of inhibition was 20.14 ± 0.96 mm for toothpaste with Ag NPs, whereas no zone of inhibition was observed with the toothpaste without Ag NPs.
Abadi et al. demonstrated the antibacterial efficacy of an alcohol-free mouthwash with a low concentration of colloidal Ag NPs (0.024–50 μg/ml).[56]
Effects on enamel and dentin
Most studies done on enamel and dentin with Ag NPs were in vitro experiments. Ag NPs can reduce the production of lactic acid in biofilm and may have the potential to reduce the demineralization of teeth.[35] Ag NPs can attach to hydroxyapatite crystals in the carious lesion.[41] Furthermore, silver ions released from Ag NPs can form insoluble silver chloride on dental hard tissue, which increases the mineral density of dental hard tissue.[15] Ag NPs can preserve exposed collagen in carious teeth by inhibiting and deactivating bacterial collagenases as well as proteinases in saliva and the dentin matrix, such as activated matrix metalloproteinases and cysteine cathepsins.[57] The preserved collagen thus acts as a scaffold for the deposition of a mineral crystal. Espíndola-Castro et al. suggested that nanosilver fluoride particles were capable of staining dentin; however, the same laboratory model concluded that brushing cycle removed the stain.[58]
Ag NPs in caries prevention
Ag NPs were combined with other nanoparticles, such as calcium glycerophosphate and zinc oxide, to produce multifunctional nanocomposites for caries prevention.[59] Ag NPs were also added into restorative materials, such as adhesives and filling resins, which can prevent secondary caries without compromising mechanical properties.[36]
Sound enamel treated with Ag NPs had a shallower lesion depth compared to enamel treated with water after biofilm challenge.[35] Besides, microhardness was increased when enamel with artificial caries was treated with Ag NPs.[57,60] The microhardness value of enamel caries treated with nanosilver fluoride was higher than that of enamel caries treated with sodium fluoride.[41]
A clinical trial reported that the mineral loss in first molars was reduced when treated with dental sealants containing Ag NPs.[44] Nanosilver fluoride also arrested dentin caries of children in two clinical trials.[39,42]
Laboratory studies claim that silver nanoparticles restrain the growth of cariogenic bacteria. Bacterial collagenase activity has been known to be impeded by Ag NPs and they also protect the collagen matrix. Therefore, Ag NPs can be useful in caries prevention. However, it is essential to prove the same with well-designed randomized clinical trials. Furthermore, staining caused by Ag Nps should be taken into consideration before clinical usage.
GOLD NANOPARTICLES
In vitro experiments
Gold (Au) is reported to have a weak antimicrobial effect against bacteria and fungi.[61-64] A combination of gold nanoparticles (Au NPs) with tetracycline or with ampicillin can improve the antibacterial activity.[65,66] Au NPs exhibit anti-inflammatory action by reducing reactive oxygen species (ROS) production by decreasing lipopolysaccharide-induced cytokine production such as interleukin (IL)-1b, IL-17, tumor necrosis factor, and modulating mitogen-activated protein kinase and phosphatidylinositol 3-kinase pathways.[67]
Hernández-Sierra et al. evaluated NPs of Ag, zinc oxide, and Au of 25 nm, 80 nm, and 125 nm average sizes. The results stated that a higher concentration of Au NPs than that of Ag NPs was required to observe bacteriostatic and bactericidal effects on S. mutans.[68] Junevičius et al.[69] compared the antimicrobial effect of toothpaste containing Ag NPs and Au. Au NPs containing toothpaste had a lower antimicrobial effect against Gram-negative bacteria when compared to Ag NPs containing toothpaste. The concentration of Au NPs required to achieve the desired effect is more compared to other nanoparticles. Furthermore, they are reported to have a weak antimicrobial effect which makes them less preferable compared to other nanoparticles used for caries prevention.
ZINC OXIDE NANOPARTICLES
Antimicrobial activity
In vitro studies
Zinc ions have demonstrated good antibacterial action which is elevated when it exists as zinc oxide nanoparticles.[70] Yamamoto found that as the particle size decreased, there was an increase in the antibacterial activity. This increase was assumed to be owed to the added H2O2 generated from the surface of ZnO.[71]
Zinc oxide nanoparticles (ZnO NP) have antibacterial properties against both Gram-positive and Gram-negative bacteria.[72] The generation of hydrogen peroxide (H2O2) from the surface of ZnO hinders bacterial growth.[71] The liberation of oxygen species on the surface of ZnO can significantly damage microorganisms.[73] Zn is effective against S. aureus,[74] Porphyromonas gingivalis and Actinomyces naeslundii,[73] Escherichia coli,[71] Streptococcus sobrinus,[16,75] and S. mutans.[76] Zinc oxide nanoparticles have photocatalytic activity and high stability.[77]
ZnO NPs in caries prevention
In vitro experiments
The inclusion of 2–5 wt% of zinc oxide nanoparticles to resin composite can provide antibacterial property without altering their properties.[78,79] The addition of ZnO and Cu nanoparticles in universal adhesive systems may provide antimicrobial activity, improve the integrity of the hybrid layer,[80] and adhesive mechanical properties.
In vivo experiments
In vivo research with Zn-containing mouth rinse has demonstrated high substantivity in the oral cavity which is active against S. mutans. The only adverse effect related to the use of zinc ions in mouth rinses is their unpleasant astringent taste. Zinc has the least tendency to stain oral tissues when compared to other active ingredients such as Ag, Sn, or chlorhexidine.[81]
Ag/ZnO nanocomposite showed enhanced antibacterial activity against S. mutans. The antibacterial mechanism involves the direct destruction of cell structure and membrane function, as well as the generation of ROS to oxidize biomacromolecules.[76] ZnONPs are effective against Gram-positive and Gram-negative bacteria in in vitro studies. In vivo studies are necessary to confirm these results. Furthermore, when added to composite, it is effective against cariogenic bacteria, but their effect on mechanical properties of composite needs to be further investigated. If used in mouth rinses, their staining property and taste should be considered.
TITANIUM DIOXIDE
In vitro studies
Titanium dioxide (TiO2) is physically and chemically stable, non-toxic, and exhibits antibacterial activity.[82] TiO2 is effective against E. coli, S. epidermidis, S. pyogenes, S. mutans, and Enterococcus faecalis.[83] TiO2 has demonstrated photocatalytic activity, with the release of ROS that attacks the bacteria from outside the cell wall.[84] TiO2 causes a lipid peroxidation reaction that subsequently collapses the cell membrane structure and therefore inhibits its functions leading to cell death.[84] TiO2 particles are used in dental composites to match the opalescence of natural teeth.[85] The addition of TiO2 to composites can improve mechanical properties.[86,87] They also demonstrated improved compressive strength when incorporated in glass ionomer cement (GIC).[88] In another study by Elsaka et al.,[89] it was reported that GI-containing 3% (w/w) TiO2 nanoparticles demonstrated superior mechanical and antibacterial properties compared to conventional GI. TiO2 is used in orthodontic composite as it is antibacterial and does not affect the shear bond strength.[90] As titanium dioxide incorporated resin composite is found to be biocompatible, it can be used as a restorative material.[91] TiO2 in the composite resin can decrease S. mutans biofilm formation over the composite resin surface.[92] TiO2 nanoparticles have proved to be effective against cariogenic bacteria. They have been demonstrated to improve the mechanical properties when added to composite and GIC. Thus, a material that can provide antibacterial property without reducing the mechanical property can be awaited in the future.
CHLORHEXIDINE
In vitro studies
Chlorhexidine (CHX) has broad-spectrum antimicrobial activities and is a widely prescribed antiplaque agent.[93,94] To overcome the rapid and uncontrolled release of free CHX from resin matrices, two methods, namely, encapsulation or nanoparticulation, are used. Nano-encapsulated particles exhibit rapid penetration and bioavailability with increased biological efficacy and decreased potential cytotoxicity.[95]
Seneviratne et al. coated CHX on mesoporous silica NPs with inner pore channels of approximately 2.5 nm. The results demonstrated that CHX NPs had antibacterial effects against both planktonic and biofilm bacteria such as Aggregatibacter actinomycetemcomitans, E. faecalis, Fusobacterium nucleatum, S. mutans, P. gingivalis, and S. sobrinus.[96] Barbour et al. developed antimicrobial chlorhexidine hexametaphosphate (CHX HMP) nanoparticles from CHX and sodium hexametaphosphate at ambient temperature and pressure.[97] There was a sustained release of CHX for more than 50 days.[97,98] Nanocarriers such as spherical poly-lactic-co-glycolic acid,[99] poly (ethylene glycol)-block-poly-(L-lactide),[100] nano-silica wires, and spheres[101-106] were studied for sustained delivery of CHX in the oral environment. A paste containing CHX HMP nanoparticles embedded into GIC has been shown to release chlorhexidine for at least 14 months.[107] A recent study demonstrated that the CHX carrier nanosystem based on iron oxide magnetic nanoparticles (IONPs) and chitosan was able to reduce biofilm formation of C. albicans and S. mutans in single or mixed cultures.[108] CHX-HMP nanoparticles aid in achieving CHX rich oral environment for a longer duration and at higher concentration compared to the conventional solution of CHX digluconate. Furthermore, the potential antibacterial effect of CHX nanoparticles aids in the treatment of biofilm-related oral diseases such as dental caries.
CHITOSAN
In vitro experiments
Chitosan nanoparticles (ChNPs) are manufactured by crosslinking methods such as ion gelation with polyanionic sodium triphosphate.[109] ChNPs are used in dental restorative materials to control oral biofilms.[110] ChNPs incorporated dental varnishes demonstrated more potent antimicrobial activity than propolis, miwask, or chlorhexidine incorporated varnishes against S. mutans.[111] Rutin (a flavonoid from plant source with antibacterial activity) loaded into ChNPs possessed higher antibacterial activity compared with pure rutin or chitosan nanoparticles alone.[112]
Covarrubias et al. demonstrated antimicrobial activity of hybrid nanoparticles comprising copper nanoparticles with a chitosan shell (CuChNP) against S. mutans. CuChNP prevented S. mutans growth on the human tooth surface as well as disrupted and killed the bacterial cells in an established dental biofilm. Chitosan also interacted with tooth hydroxyapatite and bacterial cell wall, which improved the adhesion of copper to the tooth surface and improved the anti-biofilm activity.[113] In another study, chloroaluminum phthalocyanine (ClAlPc) encapsulated in chitosan nanoparticles (ChNPs) were found to be effective against S. mutans biofilm, encouraging its use in clinical studies.[114] ChNPs exhibit good biocompatibility and antimicrobial activity against cariogenic bacteria in vitro and they can be used as potential anticariogenic agents, though further in vivo studies are necessary to establish their clinical efficacy.
HYDROXYAPATITE
In vitro experiments
Acids produced by bacterial metabolism result in mineral loss from the hard tissue in the early stages of caries attack, but the collagen network remains unaffected. HA nanoparticles (HA NPs) are used to remineralize this organic scaffold by acting either as a direct replacement of lost minerals or as a carrier for lost ions.[115] HA NPs have been integrated into products for oral care such as dentifrices and mouthwash to promote the remineralization of enamel by replacing calcium and phosphate ions in the areas from which minerals were dissolved, restoring integrity.[116] An in situ study with HA NPs incorporated toothpaste showed that HA NPs can penetrate tooth porosities and can produce a protective layer on the tooth’s surface against a carious attack.[117] HA NPs in toothpaste promote enamel regeneration by the formation of biomimetic film similar in morphology and structure to the biologic hydroxyapatite of enamel. The new layer of apatite showed resistance to toothbrushing due to the chemical bonds between the synthetic and natural crystals of enamel.[118]
Nano-HA paste showed a protective layer with globular deposits on artificially produced incipient caries-like lesions when compared to fluoride varnish and casein phosphopeptide–amorphous calcium phosphate (CPPACP).[119] A similar study showed that both nano-HA and CPP-ACP had a remineralizing effect on the early stages of caries.[120] Several in vitro studies reported the better remineralizing potential of HA NPs when compared to toothpaste containing calcium and potassium ions and sodium nitrate.[121-127]
HA NPs incorporated pit and fissure sealants demonstrated a remineralized region at sealant enamel interface. They also showed a higher degree of conversion and increased ion release.[128] Incorporation of HA NPs into dental composites promoted enamel remineralization at a potentially cariogenic pH of 4.[129]
In vivo experiments
An in vivo study showed a decrease in caries incidence by 56% in children brushing with a 5% HA NP toothpaste for 3 years.[130] The combination of nano-hydroxyapatite gel and ozone therapy was shown to remineralize initial approximal enamel and dentine subsurface lesions of posterior teeth.
However, the treatment procedures should be continued for a long time to achieve nonrestorative treatment of caries.[131] Mouthwash containing HA and Zn NPs helped in controlling bacterial biofilm formation and there was an accumulation of HA aggregates.[132-134] Nano-HA showed positive results in remineralization, making it preferable for caries prevention. Nano-HA is a relatively new material with good physical, chemical, and mechanical properties. However, its application in preventive dentistry should be investigated further.
CASEIN PHOSPHOPEPTIDE –AMORPHOUS CALCIUM PHOSPHATE (CPP-ACP)
In vitro and in vivo studies
CPP–ACP nanocomplexes decreased demineralization and enhanced remineralization of enamel by localizing at the surface of the tooth, bringing about buffering of the phosphate and calcium-free ion activities and maintaining a state of super-saturation.[135,136] They form a calcium and phosphate reservoir that is bound to plaque and dental surfaces.[137] A clinical trial conducted for 24 months demonstrated the efficacy of toothpaste containing CPP in preventing carious lesions.[138] Several in vitro and in situ studies have shown that toothpaste with CPP–ACP nano complexes prevented enamel demineralization produced by soft drinks.[139-141] Toothpaste containing CPP–ACP NPs with L. rhamnosus (probiotic strain) had effective remineralizing and antimicrobial efficiency.[142] CPP–ACP and fluoride were suggested to remineralize initial dental caries and white spot lesions. However, CPP–ACP had a slightly lower potential in the remineralization of early enamel caries compared to fluoride.[120,143-145] Studies supporting the clinical efficacy of CPP-ACP are limited and inconsistent. CPP-ACP nanocomplexes cannot be used as a substitute for fluoride or when other caries preventive interventions such as sealants and resin infiltration are available.
BIOACTIVE GLASS
In vitro studies
Bioactive glass nanoparticles (BAG NP) exhibited better remineralization potential when compared to conventional BAG due to increased surface area and higher Ca/P ratios, thus slowing the progress of dental caries.[146-148] When BAG NPs comes in contact with an aqueous solution, they will take a mesoporous shape, which allows the formation of apatite on the dentine surface. The pH rise provokes the precipitation of HA. Phosphate and calcium ions in the bioactive glass and minerals from saliva activate the mineralizing process.[149]
In vitro studies have demonstrated that BAG NPs could make dentin more acid-resistant by inducing mineral formation on dentin surfaces.[150,151] The new HA layer formed is similar to those of enamel or dentine and presents better resistance to abrasion.[152] The fluoride-containing bioactive glass had a better capacity for remineralization compared to BAG toothpaste and sodium monofluorophosphate toothpaste.[153]
BAG NPs toothpaste also demonstrated antibacterial properties.[154] BAG NPs can inhibit S. mutans biofilm.[155] BAG NPs can create an unfavorable environment for bacterial growth by the release of alkaline ions that cause an elevation in the pH. The addition of fluoride to BAG provided higher resistance to acid dissolution, allowing the formation of fluorapatite on the tooth surface.[156] This deposit of fluorapatite on the dentine surface occluded dentinal tubules and decreased permeability.[157] The main mechanisms of action of BAG NPs for caries management include antibacterial effect against cariogenic bacteria, inhibition of demineralization, and promotion of remineralization. Further research should be done to find the exact mechanism of action of bioactive glass in preventing dental caries in an intraoral environment.
CONCLUSION
Nanotechnology is relatively new and has promising potential in the development of new nanoparticles that can be used in the prevention of dental caries. Right now, oral care products such as toothpaste and mouth rinses contain NPs with antimicrobial, anti-inflammatory, and remineralizing properties. Although many NPs are used in dental restorative materials to prevent caries that are more effective than traditional materials only few which are extensively researched are highlighted in this review. Nanodentistry will be cost-effective, time-saving, and prevent the patients from future complex dental procedures. Although there are various reports with positive results in favor of nanoparticles, the clinical application of these techniques for caries prevention is limited. Further studies on dosage, viability, steps to overcome toxicity, and the performance of nanoparticles in the oral environment are necessary.
Declaration of consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Evidence-based dentistry caries risk assessment and disease management. Dent Clin North Am. 2019;63:119-28.
- [CrossRef] [PubMed] [Google Scholar]
- Bacteria of dental caries in primary and permanent teeth in children and young adults. J Clin Microbiol. 2008;46:1407-17.
- [CrossRef] [PubMed] [Google Scholar]
- Noninvasive caries risk-based management in private practice settings may lead to reduced caries experience over time. J Evid Based Dent Pract. 2016;16:239-42.
- [CrossRef] [PubMed] [Google Scholar]
- Noninvasive dentistry: A dream or reality? Caries Res. 2015;49(Suppl 1):11-7.
- [CrossRef] [PubMed] [Google Scholar]
- Fluoride and environmental health: A review. Rev Environ Sci Biotechnol. 2009;8:59-79.
- [CrossRef] [Google Scholar]
- Mucoadhesive polymers for delivery of drugs to the oral cavity. Recent Pat Drug Deliv Formul. 2008;2:108-19.
- [CrossRef] [PubMed] [Google Scholar]
- Fluoride toothpastes of different concentrations for preventing dental caries in children and adolescents. Cochrane Database Syst Rev. 2010;1:CD007868.
- [CrossRef] [PubMed] [Google Scholar]
- Nanotechnology strategies for antibacterial and remineralizing composites and adhesives to tackle dental caries. Nanomedicine (Lond). 2015;10:627-41.
- [CrossRef] [PubMed] [Google Scholar]
- Nanomaterials in preventive dentistry. Nat Nanotechnol. 2010;5:565-9.
- [CrossRef] [PubMed] [Google Scholar]
- Nanotechnology in cosmetics: Opportunities and challenges. J Pharm Bioallied Sci. 2012;4:186-93.
- [CrossRef] [PubMed] [Google Scholar]
- Advanced drug delivery systems for local treatment of the oral cavity. Ther Deliv. 2015;6:595-608.
- [CrossRef] [PubMed] [Google Scholar]
- Developing biocompatible silver nanoparticles using epigallocatechin gallate for dental use. Arch Oral Biol. 2019;102:106-12.
- [CrossRef] [PubMed] [Google Scholar]
- The use of nanoparticles to control oral biofilm formation. J Dent Res. 2010;89:1175-86.
- [CrossRef] [PubMed] [Google Scholar]
- Nanoparticles used in dentistry: A review. J Oral Biol Craniofac Res. 2018;8:58-67.
- [CrossRef] [PubMed] [Google Scholar]
- pH-activated nanoparticles for controlled topical delivery of farnesol to disrupt oral biofilm virulence. ACS Nano. 2015;9:2390-404.
- [CrossRef] [PubMed] [Google Scholar]
- Antibacterial properties of nanoparticles. Trends Biotechnol. 2012;30:499-511.
- [CrossRef] [PubMed] [Google Scholar]
- Nanotechnology-based restorative materials for dental caries management. Trends Biotechnol. 2013;31:459-67.
- [CrossRef] [PubMed] [Google Scholar]
- Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J Nanotechnol. 2018;9:1050-74.
- [CrossRef] [PubMed] [Google Scholar]
- Nano-bio interactions: Guiding the development of nanoparticle therapeutics, diagnostics, and imaging agents. Pharm Res. 2016;33:2311-3.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanical properties of visible light-cured resins reinforced with hydroxyapatite for dental restoration. Dent Mater. 2002;18:49-57.
- [CrossRef] [Google Scholar]
- Evaluation of diametral tensile strength and Knoop microhardness of five nanofilled composites in dentin and enamel shades. Stomatologija. 2006;8:67-9.
- [Google Scholar]
- Filler features and their effects on wear and degree of conversion of particulate dental resin composites. Biomaterials. 2005;26:4932-7.
- [CrossRef] [PubMed] [Google Scholar]
- Biodegradable polylactide and its nanocomposites: Opening a new dimension for plastics and composites. Macromol Rapid Commun. 2003;24:815-40.
- [CrossRef] [Google Scholar]
- Nano reinforcements of bio-based polymers-the hope and the reality. J Am Chem Soc. 2003;225:33.
- [Google Scholar]
- Role of microbial biofilms in the maintenance of oral health and in the development of dental caries and periodontal diseases. Consensus report of group 1 of the joint EFP/ORCA workshop on the boundaries between caries and periodontal disease. J Clin Periodontol. 2017;44(Suppl 18):S5-11.
- [CrossRef] [PubMed] [Google Scholar]
- Biofilm-specific antibiotic resistance. Future Microbiol. 2012;7:1061-72.
- [CrossRef] [PubMed] [Google Scholar]
- Periodontal bacterial invasion and infection: Contribution to atherosclerotic pathology. J Clin Periodontol. 2013;40(Suppl 14):S30-50.
- [CrossRef] [PubMed] [Google Scholar]
- Antimicrobial applications of nanotechnology: Methods and literature. Int J Nanomedicine. 2012;7:2767-81.
- [CrossRef] [PubMed] [Google Scholar]
- Nanoparticles as anti-microbial, anti-inflammatory, and remineralizing agents in oral care cosmetics: A review of the current situation. Nanomaterials (Basel). 2020;10:140.
- [CrossRef] [PubMed] [Google Scholar]
- Local treatment of the dental caries using nanoparticles. Biomed Pharmacother. 2018;108:443-7.
- [CrossRef] [PubMed] [Google Scholar]
- Antimicrobial efficacy of nanosilver and chitosan against Streptococcus mutans, as an ingredient of toothpaste formulation: An in vitro study. J Indian Soc Pedod Prev Dent. 2019;37:46-54.
- [CrossRef] [PubMed] [Google Scholar]
- Inhibitory effect of reduced graphene oxide-silver nanocomposite on progression of artificial enamel caries. J Appl Oral Sci. 2018;27:e20180042.
- [CrossRef] [PubMed] [Google Scholar]
- Dual antibacterial agents of nano-silver and 12-methacryloyloxydodecylpyridinium bromide in dental adhesive to inhibit caries. J Biomed Mater Res B Appl Biomater. 2013;101:929-38.
- [CrossRef] [PubMed] [Google Scholar]
- Nanosilver coated orthodontic brackets: In vivo antibacterial properties and ion release. Eur J Orthod. 2017;39:9-16.
- [CrossRef] [PubMed] [Google Scholar]
- Biosynthesis of silver nanoparticles on orthodontic elastomeric modules: Evaluation of mechanical and antibacterial properties. Molecules. 2017;22:1407.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative cariostatic efficacy of a novel Nano-silver fluoride varnish with 38% silver diamine fluoride varnish a double-blind randomized clinical trial. J Clin Exp Dent. 2019;11:e105-12.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of a new nano-silver fluoride-containing dentifrice on demineralization of enamel and Streptococcus mutans adhesion and acidogenicity. Int J Dent. 2018;2018:1351925.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of nano hydroxyapatite, nano silver fluoride and sodium fluoride varnish on primary teeth enamel remineralization: An in vitro study. J Clin Diagn Res. 2017;11:ZC97-100.
- [CrossRef] [PubMed] [Google Scholar]
- A new “silver-bullet” to treat caries in children-nano silver fluoride: A randomised clinical trial. J Dent. 2014;42:945-51.
- [CrossRef] [PubMed] [Google Scholar]
- An innovative approach to treating dental decay in children. A new anti-caries agent. J Mater Sci Mater Med. 2014;25:2041-7.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of silver nanoparticle-added pit and fissure sealant in the prevention of dental caries in children. J Clin Pediatr Dent. 2017;41:48-52.
- [CrossRef] [PubMed] [Google Scholar]
- Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl Microbiol Biotechnol. 2010;85:1115-22.
- [CrossRef] [PubMed] [Google Scholar]
- Antimicrobial efficacy of nanosilver, sodium hypochlorite and chlorhexidine gluconate against Enterococcus faecalis. Afr J Biotechnol. 2011;10:6799-803.
- [Google Scholar]
- Antibacterial effect of silver nanoparticles against four foodborne pathogens. Jundishapur J Microbiol. 2014;7:e8720.
- [CrossRef] [Google Scholar]
- Effects of antibacterial primers with quaternary ammonium and nano-silver on Streptococcus mutans impregnated in human dentin blocks. Dent Mater. 2013;29:462-72.
- [CrossRef] [PubMed] [Google Scholar]
- Silver colloid nanoparticles: Synthesis, characterization, and their antibacterial activity. J Phys Chem B. 2006;110:16248-53.
- [CrossRef] [PubMed] [Google Scholar]
- The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16:2346-53.
- [CrossRef] [PubMed] [Google Scholar]
- Antimicrobial activity of silver nanoparticles encapsulated in poly-N-isopropylacrylamide-based polymeric nanoparticles. Int J Nanomedicine. 2018;13:235-49.
- [CrossRef] [PubMed] [Google Scholar]
- Antibacterial effect of silver nanoparticles synthesized using Murraya koenigii (L.) against multidrug-resistant pathogens. Bioinorg Chem Appl. 2019;2019:4649506.
- [CrossRef] [PubMed] [Google Scholar]
- Silver nanoparticles: Partial oxidation and antibacterial activities. J Biol Inorg Chem. 2007;12:527-34.
- [CrossRef] [PubMed] [Google Scholar]
- An overview of application of silver nanoparticles for biomaterials in dentistry. Mater Sci Eng C Mater Biol Appl. 2018;91:881-98.
- [CrossRef] [PubMed] [Google Scholar]
- Antibacterial efficacy of silver nanoparticles of different sizes, surface conditions and synthesis methods. Nanotoxicology. 2011;5:244-53.
- [CrossRef] [PubMed] [Google Scholar]
- Silver nanoparticles as active ingredient used for alcohol-free mouthwash. GMS Hyg Infect Control. 2013;8:Doc05.
- [Google Scholar]
- Combining bioactive multifunctional dental composite with PAMAM for root dentin remineralization. Materials (Basel). 2017;10:89.
- [CrossRef] [PubMed] [Google Scholar]
- Dentin staining caused by nano-silver fluoride: A comparative study. Oper Dent. 2020;45:435-41.
- [CrossRef] [PubMed] [Google Scholar]
- Nanosynthesis of silver-calcium glycerophosphate: Promising association against oral pathogens. Antibiotics (Basel). 2018;7:52.
- [CrossRef] [PubMed] [Google Scholar]
- In vitro evaluation of the remineralizing potential and antimicrobial activity of a cariostatic agent with silver nanoparticles. Braz Dent J. 2017;28:738-43.
- [CrossRef] [PubMed] [Google Scholar]
- Biosynthesis, characterization and antimicrobial activity of gold nanoparticles from leaf extracts of Annona muricata. J. Nanostruct Chem. 2019;9:111-7.
- [CrossRef] [Google Scholar]
- Antibacterial activity of biosynthesized gold nanoparticles using biomolecules from Lignosus rhinocerotis and chitosan. Saudi Pharm J. 2019;27:283-92.
- [CrossRef] [PubMed] [Google Scholar]
- Antimicrobial gold nanoclusters: Recent developments and future perspectives. Int J Mol Sci. 2019;20:2924.
- [CrossRef] [PubMed] [Google Scholar]
- Advances in lipid and metal nanoparticles for antimicrobial peptide delivery. Pharmaceutics. 2019;11:588.
- [CrossRef] [PubMed] [Google Scholar]
- Green synthesis of gold nanoparticles using Fusarium oxysporum and antibacterial activity of its tetracycline conjugant. J Mycol Med. 2019;29:7-13.
- [CrossRef] [PubMed] [Google Scholar]
- Enhanced antibiotic activity of ampicillin conjugated to gold nanoparticles on PEGylated rosette nanotubes. Int J Nanomedicine. 2019;14:7281-9.
- [CrossRef] [PubMed] [Google Scholar]
- Anti-inflammatory mechanism of various metal and metal oxide nanoparticles synthesized using plant extracts: A review. Biomed Pharmacother. 2019;109:2561-72.
- [CrossRef] [PubMed] [Google Scholar]
- The antimicrobial sensitivity of Streptococcus mutans to nanoparticles of silver, zinc oxide, and gold. Nanomedicine. 2008;4:237-40.
- [CrossRef] [PubMed] [Google Scholar]
- Antimicrobial activity of silver and gold in toothpastes: A comparative analysis. Stomatologija. 2015;17:9-12.
- [Google Scholar]
- Advances in dental materials through nanotechnology: Facts, perspectives and toxicological aspects. Trends Biotechnol. 2015;33:621-36.
- [CrossRef] [PubMed] [Google Scholar]
- Influence of particle size on the antibacterial activity of zinc oxide. Int J Inorg Mater. 2001;3:643-6.
- [CrossRef] [Google Scholar]
- Nanoparticles: A promising novel adjunct for dentistry. Indian J Dent Sci. 2019;11:167-73.
- [CrossRef] [Google Scholar]
- ZnO nanoparticles inhibit the activity of Porphyromonas gingivalis and Actinomyces naeslundii and promote the mineralization of the cementum. BMC Oral Health. 2019;19:84.
- [CrossRef] [PubMed] [Google Scholar]
- Multilayered composite coatings of titanium dioxide nanotubes decorated with zinc oxide and hydroxyapatite nanoparticles: Controlled release of Zn and antimicrobial properties against Staphylococcus aureus. Int J Nanomedicine. 2019;14:3583-600.
- [CrossRef] [PubMed] [Google Scholar]
- Antibacterial activity of dental composites containing zinc oxide nanoparticles. J Biomed Mater Res B Appl Biomater. 2010;94:22-31.
- [Google Scholar]
- Antibacterial activity and mechanism of Ag/ZnO nanocomposite against anaerobic oral pathogen Streptococcus mutans. J Mater Sci Mater Med. 2017;28:23.
- [CrossRef] [PubMed] [Google Scholar]
- The synergetic antibacterial activity of Ag islands on ZnO (Ag/ ZnO) heterostructure nanoparticles and its mode of action. J Inorg Biochem. 2014;130:74-83.
- [CrossRef] [PubMed] [Google Scholar]
- Antibacterial, physical and mechanical properties of flowable resin composites containing zinc oxide nanoparticles. Dent Mater. 2013;29:495-505.
- [CrossRef] [PubMed] [Google Scholar]
- Model resin composites incorporating ZnO-NP: Activity against S. mutans and physicochemical properties characterization. J Appl Oral Sci. 2018;26:e20170270.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of zinc/copper nanoparticles on bonding to artificially caries-affected dentin. Dent Mater. 2018;34:e138.
- [CrossRef] [Google Scholar]
- Substantivity of zinc salts used as rinsing solutions and their effect on the inhibition of Streptococcus mutans. J Trace Elem Med Biol. 2007;21:92-101.
- [CrossRef] [PubMed] [Google Scholar]
- Antibacterial properties of silver doped TiO2 nanoparticles synthesized via sol-gel technique. Macromol Res. 2016;24:488-93.
- [CrossRef] [Google Scholar]
- Photocatalytic antibacterial effects are maintained on resin-based TiO2 nanocomposites after cessation of UV irradiation. PLoS One. 2013;8:e75929.
- [CrossRef] [PubMed] [Google Scholar]
- Bactericidal activity of photocatalytic TiO(2) reaction: Toward an understanding of its killing mechanism. Appl Environ Microbiol. 1999;65:4094-8.
- [CrossRef] [PubMed] [Google Scholar]
- Influence of TiO2 nanoparticles on the optical properties of resin composites. Dent Mater. 2009;25:1142-7.
- [CrossRef] [PubMed] [Google Scholar]
- Nanoparticle-reinforced resin-based dental composites. J Dent. 2008;36:450-5.
- [CrossRef] [PubMed] [Google Scholar]
- Improving performance of dental resins by adding titanium dioxide nanoparticles. Dent Mater. 2011;27:972-82.
- [CrossRef] [PubMed] [Google Scholar]
- TiO2 nanotubes improve physico-mechanical properties of glass ionomer cement. Dent Mater. 2020;36:e85-92.
- [CrossRef] [PubMed] [Google Scholar]
- Titanium dioxide nanoparticles addition to a conventional glass-ionomer restorative: Influence on physical and antibacterial properties. J Dent. 2011;39:589-98.
- [CrossRef] [PubMed] [Google Scholar]
- Shear bond strength and antibacterial effects of orthodontic composite containing TiO2 nanoparticles. Eur J Orthod. 2013;35:676-9.
- [CrossRef] [PubMed] [Google Scholar]
- Reinforcement of flowable dental composites with titanium dioxide nanotubes. Dent Mater. 2016;32:817-26.
- [CrossRef] [PubMed] [Google Scholar]
- Titanium dioxide and modified titanium dioxide by silver nanoparticles as an anti-biofilm filler content for composite resins. Dent Mater. 2019;35:e36-46.
- [CrossRef] [PubMed] [Google Scholar]
- The efficacy of 0.12% chlorhexidine versus 0.12% chlorhexidine plus hyaluronic acid mouthwash on healing of submerged single implant insertion areas: A short-term randomized controlled clinical trial. Int J Dent Hyg. 2017;15:65-72.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of chlorhexidine dentifrice or gel versus chlorhexidine mouthwash on plaque, gingivitis, bleeding and tooth discoloration: A systematic review. Int J Dent Hyg. 2015;13:83-92.
- [CrossRef] [PubMed] [Google Scholar]
- Polymeric and inorganic nanoscopical antimicrobial fillers in dentistry. Acta Biomater. 2020;101:69-101.
- [CrossRef] [PubMed] [Google Scholar]
- Nanoparticle-encapsulated chlorhexidine against oral bacterial biofilms. PLoS One. 2014;9:e103234.
- [CrossRef] [PubMed] [Google Scholar]
- Synthesis, characterization, and efficacy of antimicrobial chlorhexidine hexametaphosphate nanoparticles for applications in biomedical materials and consumer products. Int J Nanomedicine. 2013;8:3507-19.
- [CrossRef] [PubMed] [Google Scholar]
- Development of a novel antimicrobial-releasing glass ionomer cement functionalized with chlorhexidine hexametaphosphate nanoparticles. J Nanobiotechnology. 2014;12:3.
- [CrossRef] [PubMed] [Google Scholar]
- PLGA nanoparticles as chlorhexidine-delivery carrier to resin-dentin adhesive interface. Dent Mater. 2017;33:830-46.
- [CrossRef] [PubMed] [Google Scholar]
- Synthesis and characterization of new chlorhexidine-containing nanoparticles for root canal disinfection. Materials (Basel). 2016;9:452.
- [CrossRef] [PubMed] [Google Scholar]
- pH-responsive release of chlorhexidine from modified nanoporous silica nanoparticles for dental applications. Bionanomaterials. 2016;17:59-72.
- [CrossRef] [Google Scholar]
- The spherical nanoparticle-encapsulated chlorhexidine enhances anti-biofilm efficiency through an effective releasing mode and close microbial interactions. Int J Nanomedicine. 2016;11:2471-80.
- [CrossRef] [PubMed] [Google Scholar]
- Synergistic bactericidal activity of chlorhexidine-loaded, silver-decorated mesoporous silica nanoparticles. Int J Nanomedicine. 2017;12:3577-89.
- [CrossRef] [PubMed] [Google Scholar]
- Redox/pH dual-controlled release of chlorhexidine and silver ions from biodegradable mesoporous silica nanoparticles against oral biofilms. Int J Nanomedicine. 2018;13:7697-709.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of chlorhexidine-encapsulated mesoporous silica nanoparticles on the anti-biofilm and mechanical properties of glass ionomer cement. Molecules. 2017;22:1225.
- [CrossRef] [PubMed] [Google Scholar]
- Nanoparticles for controlled delivery and sustained release of chlorhexidine in the oral environment. Oral Dis. 2015;21:641-4.
- [CrossRef] [PubMed] [Google Scholar]
- Glass ionomer cements functionalised with a concentrated paste of chlorhexidine hexametaphosphate provides dose-dependent chlorhexidine release over at least 14 months. J Dent. 2016;45:53-8.
- [CrossRef] [PubMed] [Google Scholar]
- Antibiofilm effect of chlorhexidine-carrier nanosystem based on iron oxide magnetic nanoparticles and chitosan. Colloids Surf B Biointerfaces. 2019;174:224-31.
- [CrossRef] [PubMed] [Google Scholar]
- Fabrication of letrozole formulation using chitosan nanoparticles through ionic gelation method. Int J Biol Macromol. 2017;104:1820-32.
- [CrossRef] [PubMed] [Google Scholar]
- Chitosan biomaterials for current and potential dental applications. Materials (Basel). 2017;10:602-21.
- [CrossRef] [PubMed] [Google Scholar]
- Antibacterial activity against Streptococcus mutans and inhibition of bacterial induced enamel demineralization of propolis, miswak, and chitosan nanoparticles based dental varnishes. J Adv Res. 2017;8:387-92.
- [CrossRef] [PubMed] [Google Scholar]
- Rutin-chitosan nanoparticles: Fabrication, characterization and application in dental disorders. Polym Plast Technol Eng. 2015;54:202-8.
- [CrossRef] [Google Scholar]
- Synthesis of hybrid copper-chitosan nanoparticles with antibacterial activity against cariogenic Streptococcus mutans. Dent Mater J. 2018;37:379-84.
- [CrossRef] [PubMed] [Google Scholar]
- Conjugate of chitosan nanoparticles with chloroaluminium phthalocyanine: Synthesis, characterization and photoinactivation of Streptococcus mutans biofilm. Photodiagnosis Photodyn Ther. 2020;30:101709.
- [CrossRef] [PubMed] [Google Scholar]
- Remineralization potential of fully demineralized dentin infiltrated with silica and hydroxyapatite nanoparticles. Dent Mater. 2014;30:249-62.
- [CrossRef] [PubMed] [Google Scholar]
- Safety assessment of nano-hydroxyapatite as an oral care ingredient according to the EU cosmetics regulation. Cosmetics. 2018;5:53.
- [CrossRef] [Google Scholar]
- Effect of an experimental paste with hydroxyapatite nanoparticles and fluoride on dental demineralisation and remineralisation in situ. Caries Res. 2015;49:499-507.
- [CrossRef] [PubMed] [Google Scholar]
- Enamel remineralization and repair results of biomimetic hydroxyapatite toothpaste on deciduous teeth: An effective option to fluoride toothpaste. J Nanobiotechnology. 2019;17:17.
- [CrossRef] [PubMed] [Google Scholar]
- In vitro effects of nano-hydroxyapatite paste on initial enamel carious lesions. Pediatr Dent. 2014;36:85-9.
- [Google Scholar]
- Comparative evaluation of nano-hydroxyapatite and casein phosphopeptide-amorphous calcium phosphate on the remineralization potential of early enamel lesions: An in vitro study. J Orofac Sci. 2017;9:28-33.
- [CrossRef] [Google Scholar]
- Comparing the effectiveness of four desensitizing toothpastes on dentinal tubule occlusion: A scanning electron microscope analysis. J Conserv Dent. 2017;20:269-72.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of nanohydroxyapatite toothpaste on microhardness ofartificial carious lesions created on extracted teeth. J Dent Res Dent Clin Dent Prospects. 2017;11:14-7.
- [CrossRef] [PubMed] [Google Scholar]
- In vitro effects of hydroxyapatite containing toothpastes on dentin permeability after multiple applications and ageing. Sci Rep. 2018;8:4888.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of nano-hydroxyapatite toothpaste on enamel surface remineralization. An in vitro study. Am J Dent. 2014;27:287-90.
- [Google Scholar]
- Enamel and dentine remineralization by nano-hydroxyapatite toothpastes. J Dent. 2011;39:430-7.
- [CrossRef] [PubMed] [Google Scholar]
- Protective effects of a zinc-hydroxyapatite toothpaste on enamel erosion: SEM study. Ann Stomatol (Roma). 2017;7:38-45.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative evaluation of surface microhardness of artificially demineralized human enamel with nano hydroxyapatite, calcium phosphate, and potassium nitrate remineralizing agents: An in vitro study. Conserv Dent Endod J. 2018;3:50-5.
- [CrossRef] [Google Scholar]
- Evaluation of remineralization potential and mechanical properties of pit and fissure sealants fortified with nano-hydroxyapatite and nano-amorphous calcium phosphate fillers: An in vitro study. J Conserv Dent. 2018;21:681-90.
- [CrossRef] [PubMed] [Google Scholar]
- Fabrication and characterization of remineralizing dental composites containing hydroxyapatite nanoparticles. J Mech Behav Biomed Mater. 2020;109:103817.
- [CrossRef] [PubMed] [Google Scholar]
- Effect to apatite-containing dentifrices on dental caries in school children. J Dent Health. 1989;39:104-9.
- [CrossRef] [Google Scholar]
- Effect of nano-hydroxyapatite and ozone on approximal initial caries: A randomized clinical trial. Sci Rep. 2020;10:11192.
- [CrossRef] [PubMed] [Google Scholar]
- Influence of a mouthwash containing hydroxyapatite microclusters on bacterial adherence in situ. Clin Oral Investig. 2013;17:805-14.
- [CrossRef] [PubMed] [Google Scholar]
- Antiplaque and remineralizing effects of biorepair mouthwash: A comparative clinical trial. Pediatr Dent J. 2016;26:89-94.
- [CrossRef] [Google Scholar]
- Efficacy of a mouthrinse based on hydroxyapatite to reduce initial bacterial colonisation in situ. Arch Oral Biol. 2017;80:18-26.
- [CrossRef] [PubMed] [Google Scholar]
- Remineralization of enamel subsurface lesions with casein phosphopeptide-amorphous calcium phosphate: A quantitative energy dispersive X-ray analysis using scanning electron microscopy: An in vitro study. J Conserv Dent. 2012;15:61-7.
- [CrossRef] [PubMed] [Google Scholar]
- Inhibition of dental erosion by casein and casein-derived proteins. Caries Res. 2011;45:13-20.
- [CrossRef] [PubMed] [Google Scholar]
- Protective effect of casein phosphopeptide-amorphous calcium phosphate on enamel erosion: Atomic force microscopy studies. Scanning. 2015;37:327-34.
- [CrossRef] [PubMed] [Google Scholar]
- Study of the efficacy of toothpaste containing casein phosphopeptide in the prevention of dental caries: A randomized controlled trial in 12-to 15-year-old high caries risk children in Bangalore, India. Caries Res. 2009;43:430-5.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of two toothpastes on repairing enamel erosion produced by a soft drink: An AFM in vitro study. J Dent. 2010;38:868-74.
- [CrossRef] [PubMed] [Google Scholar]
- Analysis of dentin/enamel remineralization by a CPP-ACP paste: AFM and SEM study. Scanning. 2013;35:366-74.
- [CrossRef] [PubMed] [Google Scholar]
- Surface remineralization potential of casein phosphopeptide-amorphous calcium phosphate on enamel eroded by cola-drinks: An in situ model study. Contemp Clin Dent. 2013;4:331-7.
- [CrossRef] [PubMed] [Google Scholar]
- Antibacterial and remineralization efficacy of casein phosphopeptide, glycomacropeptide nanocomplex, and probiotics in experimental toothpastes: An in vitro comparative study. Eur J Dent. 2019;13:391-8.
- [CrossRef] [PubMed] [Google Scholar]
- Anticariogenic complexes of amorphous calcium phosphate stabilized by casein phosphopeptides: A review. Spec Care Dentist. 1998;18:8-16.
- [CrossRef] [PubMed] [Google Scholar]
- Remineralization effect of CPP-ACP and fluoride for white spot lesions in vitro. J Dent. 2014;42:1592-602.
- [CrossRef] [PubMed] [Google Scholar]
- Enamel remineralization assessment after treatment with three different remineralizing agents using surface microhardness: An in vitro study. J Conserv Dent. 2014;17:49-52.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of the remineralizing effects of sodium fluoride and bioactive glass using bioerodible gel systems. J Dent Res Dent Clin Dent Prospects. 2009;3:117-21.
- [Google Scholar]
- Remineralization of human dentin using ultrafine bioactive glass particles. Acta Biomater. 2007;3:936-43.
- [CrossRef] [PubMed] [Google Scholar]
- Biomimetic regulation of dentine remineralization by amino acid in vitro. Dent Mater. 2019;35:298-309.
- [CrossRef] [PubMed] [Google Scholar]
- Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2013;9:4457-86.
- [CrossRef] [PubMed] [Google Scholar]
- Mineral formation on dentin induced by nano-bioactive glass. Chin Chem Lett. 2016;27:1509-14.
- [CrossRef] [Google Scholar]
- Comparative evaluation of combined remineralization agents on demineralized tooth surface. Niger J Clin Pract. 2019;22:1546-52.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of common dental materials used in preventive or operative dentistry on dentin permeability and remineralization. Oper Dent. 2011;36:222-30.
- [CrossRef] [PubMed] [Google Scholar]
- Title: Efficacy of a novel fluoride containing bioactive glass based dentifrice in remineralizing artificially induced demineralization in human enamel. Fluoride. 2019;52:447-55.
- [Google Scholar]
- Mechanisms of bioactive glass on caries management: A review. Materials (Basel). 2019;12:4183.
- [CrossRef] [PubMed] [Google Scholar]
- Antimicrobial effects of a bioactive glass combined with fluoride or triclosan on Streptococcus mutans biofilm. Arch Oral Biol. 2015;60:1059-65.
- [CrossRef] [PubMed] [Google Scholar]
- A polarized light microscopic study to comparatively evaluate four remineralizing agents on enamel viz CPP-ACPF, ReminPro, SHY-NM and colgate strong teeth. Int J Clin Pediatr Dent. 2015;8:42-7.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment of enamel remineralisation after treatment with four different remineralising agents: A scanning electron microscopy (SEM) study. J Clin Diagn Res. 2017;11:ZC136-41.
- [CrossRef] [PubMed] [Google Scholar]






