Pathogenesis of salivary gland neoplasms: The concepts of histogenesis and morphogenesis

*Corresponding author: Sanpreet Singh Sachdev, Department of Oral Pathology and Microbiology, Government Dental College and Hospital, Mumbai, India. sunpreetss@yahoo.in
-
Received: ,
Accepted: ,
How to cite this article: Sonawane SG, Sachdev SS, Sardar MA, Chettiankandy TY, Nayyar V, Ankit A. Pathogenesis of salivary gland neoplasms: The concepts of histogenesis and morphogenesis. J Global Oral Health 2023;6:59-65.
Abstract
Many features overlap between different salivary gland neoplasms (SGNs) and as a result, classifying them distinctly has always been challenging. The differences in pathogenesis give rise to variations in the histopathological morphology of the SGNs. More accurate classification of SGNs can be made if the underlying pathogenesis is adequately understood in these terms. The present review aims to elaborate on the classification of SGNs based on the concepts of histogenesis and morphogenesis. In tumor pathology, histogenesis corresponds to the origin of the neoplastic cells; whereas morphogenesis refers to the development of the shape of an organ. The type of cell in which neoplastic transformation has occurred governs the events that follow the initiation of the multistage process which results in neoplasia. Our review elucidates the pathogenesis of the salivary gland tumor to understand the resulting histopathology, tumor morphology, and cellular differentiation of the tumor which reflects the parent cell. It comprehensively covers histogenesis, morphogenesis, and their relevance to the common salivary gland tumors, along with a brief account of the immunohistochemical markers.
Keywords
Salivary gland cancer
Pleomorphic adenoma
Mucoepidermoid carcinoma
Immunohistochemistry
Myoepithelial cells
INTRODUCTION
Salivary glands are structures unique to the oral cavity and are thus of great importance to oral and maxillofacial pathologists. The different tumors derived from the elements of the salivary glands exhibit considerable cytological as well as an architectural morphologic variation under the microscope. Many features overlap between different salivary gland neoplasms (SGNs) and as a result, classifying them distinctly has always been challenging.
Proper classification in SGNs is crucial as the different tumors exhibit much variation in response to different types of therapies such as radiation or chemotherapy. The earliest well-known classification of SGNs was given by Foote and Frazell in 1954. Ever since the 2nd edition of the WHO classification of Head and Neck tumors (1991), the classification of SGNs has undergone significant updates.[1]
While most of the entities have withstood the test of time, some have been modified or deleted from their respective groups and some new entities have emerged. The recent 5th Edition of the WHO classification has also added some new entities such as intercalated duct adenoma, striated duct adenoma, microsecretory adenocarcinoma, and microcystic adenocarcinoma.[2]
These modifications are a result of generous efforts by researchers to understand the underlying mechanism for the formation of SGNs. As more clarity is achieved regarding the pathogenesis of these tumors, corresponding modifications are being made in the standard classification system.
“Histogenesis” and “Morphogenesis” are the most crucial aspects, based on which the earlier classification systems have been developed.[3] Histogenesis (Ancient Greek: histos – web, tissue; genesis – production) refers to the development of tissues from the undifferentiated cells of the germ layers of the embryo. In tumor pathology, it corresponds to the origin of the neoplastic cells. Whereas, morphogenesis (Greek: morphe – form; genesis – to produce) refers to the development of the shape of an organ. It refers to the ultimate form particular to that organ attained to which other members of the species approximate. The differences in pathogenesis give rise to variations in the histopathological morphology of the SGNs.[4,5] More accurate classification of SGNs can be made if the underlying pathogenesis is adequately understood in these terms.
The present review aims to elaborate on the classification of SGNs based on their histogenesis and morphogenesis. It would serve the objectives of providing a clearer idea to the pathologists regarding the derivation of the tumors and differentiating them in a more understandable manner. It would also offer insights into the reasons behind the peculiar morphological picture exhibited by the different SGNs.
PATHOGENESIS OF SGNs
The development of salivary glands closely resembles the branching type of morphogenesis which is also noted in other organs such as the lungs, pancreas, and kidney. The ectoderm from the initial stomodeal thickening gives rise to the parotid gland, while the submandibular and sublingual glands develop from the endodermal layer.[4] Besides these, minor salivary glands develop in the submucosa of most of the intraoral sites including the buccal mucosa, tongue, floor of the mouth, and palate.
Confirmatory diagnoses for SGNs require standards that define the limits for each subtype. There are different approaches to conceptualizing the development and differentiation of the variants of SGNs. Two main concepts are currently in vogue, histogenesis, and morphogenesis.
The etiological factors of human SGNs remain obscure except for radiation and certain viruses. Epstein–Barr virus DNA sequences have been detected in malignant lymphoepithelial lesions and Warthin’s tumor. The previous radiation and previous primary cancer have been established as risk factors for major salivary gland carcinoma. Equally important in terms of salivary gland tumorigenesis are observations of in situ carcinoma involving epithelium within lobules adjacent to established neoplasms in these glands.
The “histogenesis” concept
This concept of the development of SGNs is based on the putative cells of origin involved in the formation of specific structures of the fetal salivary gland system. The histogenic concept of salivary gland tumors has usually relied on histologic observations of the fetal salivary gland and the cellular differentiation involved in particular segments of the duct system.
Over the years, several theories involving the histogenesis concept in SGNs have been put forth:[3,6,7]
-
Basal reserve cell theory (Eversole, 1971):
Also called as “progenitor cell” theory. It is based on the hypothesis that the basal cells function as reserve cells that eventually give rise to its derivates of the luminal layer of the intercalated and the excretory ducts. The hypothesis was formulated after observation of the embryonic palatal minor salivary glands and bilayered major ducts in the human fetal salivary gland. The tumors that most appropriately fit into the hypothesis include pleomorphic adenoma, basal cell adenoma, and canalicular adenoma.
-
Pluripotent unicellular reserve cell theory (Batsakis, 1977):
This theory is another type of reserve cell theory; however, it states that only the basal cells of the excretory duct are responsible for the development of all the other salivary gland structures. The theory eliminated any implication of the intercalated duct reserve cells in the development of salivary gland units or neoplasms.
-
Semi-pluripotent bicellular reserve cell theory (Dardick, 1981):
This theory is a more elaborative and refined version of the reserve cell theories. The basal cells of the intercalated and excretory ducts were still implicated in the development of luminal structures; however, a distinction was made between the derivatives of the two types. The first type, basal cells of the intercalated ducts, gives rise to acini and the luminal cells of the intercalated and striated ducts. The second type of basal cells, excretory duct reserve cells, give rise to squamous or mucin-producing columnar cells.[6,7]
Based on this theory, it could be interpreted that mucoepidermoid carcinoma and squamous cell carcinoma arose from the excretory duct reserve cells, whereas the intercalated duct reserve cells formed all the other tumors.
It was also pointed out that the myoepithelial cells were responsible, in part, for the morphologic diversity of the neoplasms. Although the “bicellular theory of origin” has been well accepted, there has been little or no direct evidence to support the hypothesis.
-
Multicellular histogenesis concept (Dardick et al., 1989):
The theory suggests that the differentiated luminal and abluminal cells of the mature salivary glands are capable of proliferating into neoplasms [Figure 1]. It was demonstrated that the basal cells of the ducts, luminal cells, as well as acinar cells were capable of DNA synthesis and mitosis. In adult rats, when hyperplasia was induced in the salivary glands, it was found that more acinar cells were found in the S-phase of the cell cycle as compared to the intercalated ductal cells. It was, thus, evident from these observations that the proliferative compartment in the salivary glands was not restricted only to the ductal basal cells.[6,7]
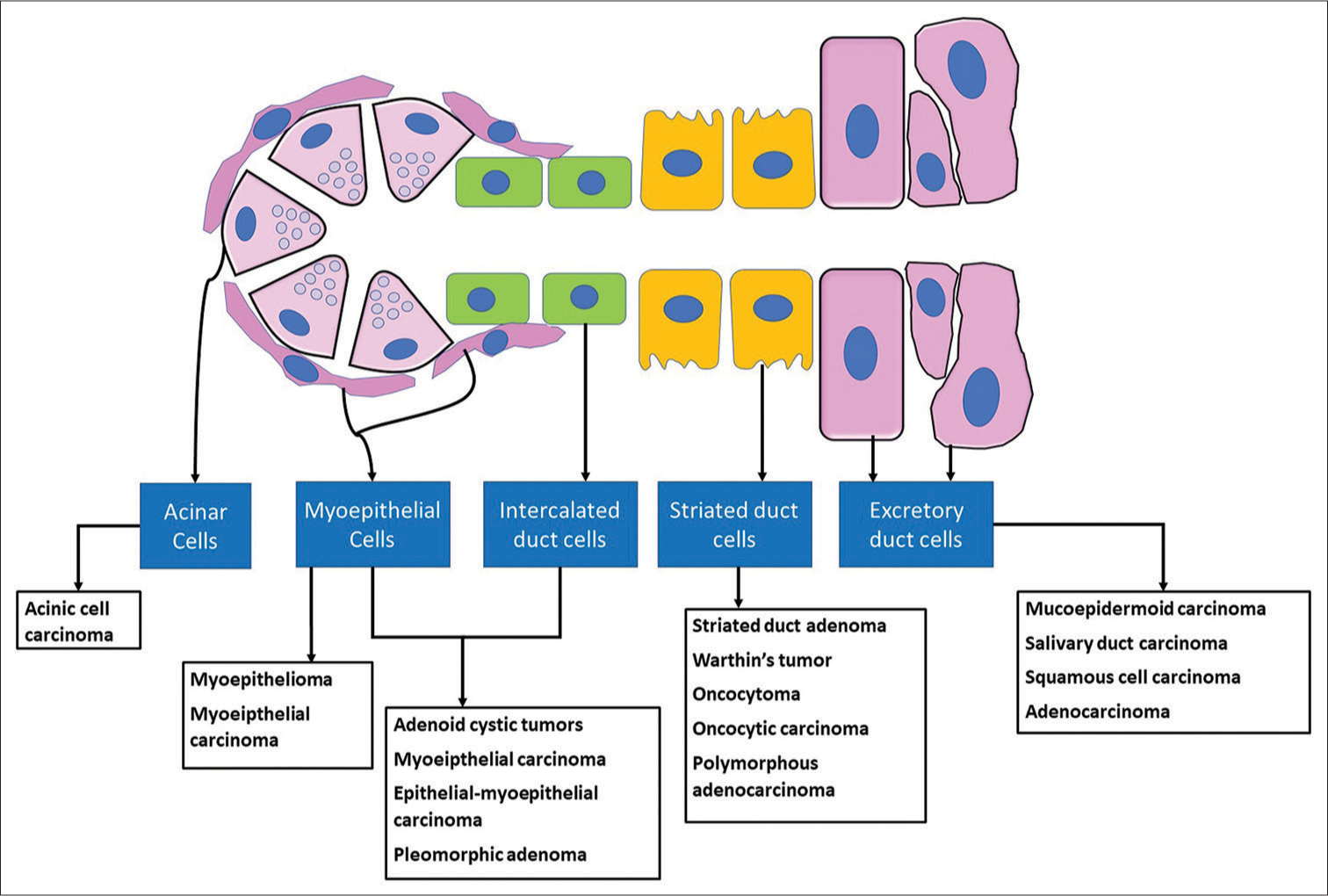
- Multicellular histogenesis concept of salivary gland neoplasms.
In terms of tumor induction, it should be appreciated that “differentiated cells are capable of metaplastic alterations’ epidermoid metaplasia has been demonstrated in acinar and myoepithelial cells of the salivary gland of the rat and secretory cells of hamster tracheal mucosa. The role of repair and replenishment could be assumed only by uncommitted stem (reserve cell) cells and by inference such as cells are solely at risk for neoplastic induction. Epithelialmyoepithelial carcinoma and the newly described entities of intercalated duct adenoma and striated duct adenoma are aptly supported by this hypothesis.
The “Morphogenesis” concept
Studies have revealed the previously unrecognized complexity of the salivary ductal epithelium and some aspects of the cellular organization of these ducts. The morphogenetic approach focuses on tumor differentiation and other cellular processes that critically influence histomorphology. They are unconcerned with the tumor initiation processes, even though these are of primary importance and are likely to influence many aspects of tumor development. The morphogenic process determines the specific features allowing recognition of each normal organ as well as differentiation of SGNs.[3,6]
The importance of this concept is that it relates the histology of the neoplastic processes directly to the classification of SGNs rather than designing a special cell of origin that are generally impossible to precisely identify once the tumor is clinically overt, thus, having better practical applications compared to the histogenesis concepts. Understanding the ductoacinar concept helps to appreciate the evolution of histological features in salivary gland tumors, which is essential for proper morphological classification.
NEOPLASTIC LUMINAL EPITHELIUM
Luminal cells either alone or in conjunction with abluminal cells are integral to many SGNs. The degree of formation, organization, and type of luminal cells greatly influences final histomorphology. In some tumors, the cytology of luminal cells may be relatively uniform, while in others such as mucoepidermoid carcinoma, variable mixtures of different ductal and/or mucin-producing acinar cells occur. Metaplastic changes may also be noted in some neoplasms. Differentiation characteristics of luminal cells influence both the development of criteria for diagnosis of any particular tumor type and the assessment of a particular case for inclusion within a specific salivary gland tumor subtype.[3,7]
NEOPLASTIC MYOEPITHELIUM
SGNs are capable of bidirectional differentiation, luminal, and abluminal, with each line giving rise to two or more cell types. The neoplastic equivalent of the normal myoepithelial cell has been an accepted participant in the histomorphology of different SGNs.[3] However, there have been arguments among different investigators concerning the identification of myoepithelial cells in SGNs. Therefore, defining neoplastic myoepithelium becomes critical to the classification of SGNs. A general requirement has been to demonstrate the presence of myofilaments or muscle-specific actin by electron microscopy and immunocytochemistry, respectively.[8] For practical purposes, the abluminal cells in SGNs can continue to be referred to as myoepithelial cells realizing that they may have little or no synthesis of muscle-type actin and thus can be the equivalent of either basal or myoepithelial cells, as well as some neoplastic hybrid of these two cell types.
The presence or absence of luminal and abluminal cells, myoepithelial cells, and extracellular matrix (ECM) consisting of proteoglycans, laminin, and elastin, form the basis of the morphogenic concept of SGN pathogenesis.[9] A permutation of these elements yields five types of combinations noted in the SGNs:
Tumors derived exclusively from luminal/acinar cells
Tumors derived exclusively from abluminal cells
Tumors derived from abluminal cells and having an ECM component
Bidirectional differentiation with an ECM component
Bidirectional differentiation without an ECM component.
The taxonomic classification of SGNs is based on the morphogenic theory of pathogenesis [Table 1].[10]
| Classification | Subclassification | Specific neoplasm | |
|---|---|---|---|
| Benign | Malignant | ||
| Luminal and myoepithelial cells | With apparent proteoglycan and basal lamina production | Pleomorphic adenoma, Basal cell adenoma | Carcinoma ex pleomorphic adenoma Adenoid cystic carcinoma Basal cell adenocarcinoma, Polymorphous adenocarcinoma Mucoepidermoid carcinoma Epithelial myoepithelial carcinoma |
| Lacking obvious proteoglycan and basal lamina production | Basal cell adenoma, Pleomorphic adenoma, Warthin’s tumor | ||
| Primarily myoepithelial/basal cells | Myoepithelioma | Myoepithelial carcinoma | |
| Primarily of luminal/acinar cells | Canalicular adenoma, ductal papilloma, cystadenoma, oncocytoma | Acinic cell carcinoma, salivary duct carcinoma, Adenocarcinoma, Oncocytic carcinoma, intercalated duct adenoma, striated duct adenoma |
|
| Undifferentiated cells | Undifferentiated carcinoma | ||
CORRELATION OF THE CONCEPTS WITH THE PATHOGENESIS OF SOME COMMON SGNs
With the understanding of the overall concepts of histogenesis and morphogenesis, it is only rational to correlate them with tumor pathogenesis. The following text discusses a brief account of this correlation noted in some of the common SGNs.
Pleomorphic adenoma
The term “pleomorphic adenoma” suggested by Willis characterizes closely the unusual histologic pattern of the lesion. The neoplastic cells exhibit an ability to differentiate into epithelial (luminal and abluminal) cells and mesenchymal (chondroid, myxoid, and osseous) cells. Many theories have been put forward in explaining the histogenesis of this bizarre tumor.[3,11]
At present, these are centered around myoepithelial cells and a reserve cell in the intercalated duct. Regezi and Batsakis postulated that the intercalated duct reserve cells differentiate into ductal and myoepithelial cells and later, in turn, can exhibit metaplasia into mesenchymal phenotype as demonstrated by the expression of vimentin and smooth muscle actin. Hubner, and more recently, Brodetskyi et al. suggested that these myoepithelial cells are responsible for the production of the fibrous, mucinous, chondroid, and osseous tissue accounting for the histomorphologic diversity of the tumor.[11]
Even so, the role of the myoepithelial cell in the histogenesis of PA is uncertain and it may only be a passive participant in the process. Dardick et al. questioned the role of intercalated duct (ICD) cells and myoepithelial cells. They stated that a neoplastically altered epithelial cell with the potential for multidirectional differentiation may be responsible for the PA.[6]
Warthin’s tumor
Warthin’s tumor is the second most common tumor in the salivary glands, almost invariably occurring in the parotid gland. This tumor was first recognized by Albrecht in 1910 and later described by Warthin in 1929.[12] The histologic appearance leaves no doubt that Warthin’s tumor differentiates both basal and luminal cells in the development of its characteristic epithelium. Unlike either normal luminal or basal/myoepithelial cells, both cell types in Warthin’s tumor are packed with mitochondria and, therefore, show oncocytic differentiation.
Numerous theories have been advanced to account for the peculiar histomorphology of this tumor. Thompson and Bryant in their study described that tumors with typical epithelial elements showed no evidence of normal lymph node architecture, and they suggested that it involved a neoplastic proliferation of parotid ductal epithelium and concomitant secondary formation of lymphoid tissue. Histochemical investigations have indicated that Warthin’s tumor most likely arose from salivary ducts in the lymphoid stroma.[13]
It was also suggested that Warthin’s tumor is most likely a delayed hypersensitivity disease, the lymphocytes being an immune reaction to the salivary ducts which undergo oncocytic change. Recent immunohistochemistry (IHC)-based studies have suggested that the lymphoid component of the tumor is an exaggerated secretory immune response. The currently accepted theory is that the tumor arises in salivary gland tissue entrapped within paraparotid or intraparotid lymph nodes during embryogenesis.
Acinic cell carcinoma (ACC)
Neoplastic acinar cells constitute an integral component of ACC.[13] In most cases of the microcystic variant of ACC, what have been referred to as vacuoles within the tumor cells, implying a degenerative process in this tumor, are in reality smaller versions of true intercellular lumens.[14]
Oncocytoma and oncocytic adenocarcinoma
The name “oncocytoma” is derived from the resemblance of these tumor cells to apparently normal cells which have been termed “oncocytes.”[15] These cells are not only limited to salivary glands but may also be found in the breast, thyroid, parathyroid, pituitary, and other glands. The phenomenon of “oncocytosis” occurs as a result of an aberrant increase in the number of mitochondria in the cells. There may represent age-related changes or occur as a result of an increase in the metabolic activity of the cells.[6]
In addition to luminal cell differentiation in oncocytomas, myoepithelial-type cells have been reported ultrastructurally, such dual differentiation is theoretically possible and would not differ from the neoplastic myoepithelium shown in otherwise classical ACC.
Adenoid cystic carcinoma
Emulation of the ductoacinar unit is complete in three model diagrams representing the recognized growth patterns, cribriform, tubular, and solid.[6,13]
In the cribriform variant, some balance exists between the differentiation of luminal cells-forming ductal structures-and basal/myoepithelial cells.[13]
Tubular variant results when luminal cells forming ducts are surrounded by a single to a few layers of basal/myoepithelial cells without intercellular materials accumulating.
The solid variant develops from an exuberant proliferation of neoplastic basal/myoepithelial cells.
Mucoepidermoid carcinoma
Tumor cell differentiation based on the organization of the normal ductoacinar unit results in a variety of cell types and a considerable spectrum of histology in mucoepidermoid carcinoma. The differentiation potential of luminal cells can produce goblet cells, non-descript cuboidal to columnar cells, glycogen-laden clear cells, and squamous cells.[16] Similarly, differentiation in abluminal cells can be seen as myoepithelial cells, intermediate cells, squamous cells, glycogen-rich clear cells, and non-specific basal cells. By mixing the cell types, it is possible to develop the range of histology both within and between cases of mucoepidermoid carcinoma, as well as various grades of the tumor.
Epithelial-myoepithelial carcinoma
As the name suggests, the tumor exhibits a bidifferential cell population comprising epithelial as well as myoepithelial cells. In this case, an apparent “inverse ductal” arrangement is noted, wherein the central luminal cells are small with eosinophilic cytoplasm and the outer myoepithelial cells appear clear due to their rich glycogen content.[17] Histogenically, the tumor may exhibit an epithelial-dominant or a myoepithelial-dominant picture or an almost equal proportion of both as noted in the classic variant. Solid, tubular, cribriform, and papillary architectures add to the histomorphologic spectrum of epithelial-myoepithelial carcinoma.
Polymorphous adenocarcinoma
The tumor exhibits a cytologically uniform yet architecturally diverse histopathological picture; thus, the name “polymorphous.” The tumor is also called “terminal duct carcinoma” indicating the putative cells of origin to be from the terminal excretory ducts.[18] The myriad histologic patterns result from the controlled development of tumor cells with varying arrangements from region to region, as well as variation between individual cases. In some examples, this consists of regions composed exclusively or predominantly of luminal cells; in other cases, as a combination of luminal and abluminal cells while in some cases, largely as basal/ myoepithelial cells.[6] Depending on the location and amounts of extracellular materials, histopathology may exhibit myxoid stroma and/or areas with a cribriform growth pattern. Combinations of these cell types and growth patterns commonly occur in individual cases.[13]
The advent of IHC has confirmed much of the assumptions on which the histogenic and morphogenic concepts of SGN pathogenesis were based. These are summarized in brief in [Table 2].[19-21]
| Neoplasm | Immunohistochemical Markers | Expression | Utility in SGNs |
|---|---|---|---|
| Pleomorphic adenoma | SMA CK7 CK8 CK13 CK14 CK17 CK18 | Both luminal and abluminal cells | Epithelial marker; differential diagnosis between myoepithelioma/myoepithelial carcinoma or “undifferentiated carcinoma” and non-epithelial tumors |
| GFAP, PLAG1, SOX10, S100, vimentin | Myoepithelial cells (variable) | Myoepithelial marker (low sensitivity) | |
| Polymorphous adenocarcinoma | EMA, S100 protein, Vimentin, Galectin-3+ve | Abluminal and luminal cells | Ductal (luminal) cell marker; apical staining pattern; |
| Acinic cell carcinoma | α-Amylase SOX10 Maspin−ve |
Acinar cells | Low sensitivity |
| Salivary duct carcinoma | Gross cystic disease fluid protein-15 | Luminal cells | Low specificity |
| Oncocytoma and oncocytic adenocarcinoma | CD117/c-Kit E-cadherin S100 PAX8 Pancytokeratin |
Striated duct cells | Strongly positive |
| Carcinoma ex pleomorphic adenoma | HER2/neu | Negative to weakly positive in ductal cells | Highly overexpressed in salivary duct carcinoma |
| Adenoid cystic carcinoma | c-Kit, Calponin, SMA, CK7 | Luminal cells | Ductal (luminal) cell marker |
| Myoepithelioma | SMA, S100, GFAP | Myoepithelial cells | High specificity, very useful |
| Calponin | Myoepithelial cells | High specificity, very useful | |
| p63 | Myoepithelial and basal cells | Myoepithelial marker, also positive for basal and squamous epithelial cells | |
| Vimentin | Myoepithelial cells | Myoepithelial marker (good for screening, low specificity) | |
| S-100 protein, SOX10 | Variable | Myoepithelial marker | |
| Mucoepidermoid carcinoma | Strong expression of PCNA, p53, and EGFR TNFa Positive for pAKT CK14 and p63+ve p63, MUC1, MUC4, MUC5AC and MUC5B |
Myoepithelial and basal cells | Myoepithelial marker, also positive for basal and squamous epithelial cells |
GFAP: Glial fibrillary acidic protein, SMA: α-Smooth muscle actin, EMA: Epithelial membrane antigen, SGNs: Salivary gland neoplasms, PLAG1: Pleomorphic adenoma gene-1; PCNA: Proliferating cell nuclear antigen; EGFR: Epidermal Growth Factor Receptor; TNF: Tumor Necrosis Factor
CONCLUSION
The present review elucidates the pathogenesis of the salivary gland tumor to understand the resulting histopathology, tumor morphology, and cellular differentiation of the tumor which reflect the parent cell. The type of cell in which neoplastic transformation has occurred governs the events that follow the initiation of the multistage process which results in neoplasia. This influences the biology of the tumor and the pattern of cellular differentiation within it. It is important for the pathologist to assess the cytoarchitectural features and cytoarchitectural profile of these neoplasms and correlate them with histogenesis for better understanding which in turn will help in diagnosis and management.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Salivary gland tumours: Diagnostic challenges and an update on the latest WHO classification. Diagn Histopathol. 2020;26:147-58.
- [CrossRef] [Google Scholar]
- Update from the 5th edition of the World Health Organization classification of head and neck tumors: Salivary glands. Head Neck Pathol. 2022;16:40-53.
- [CrossRef] [PubMed] [Google Scholar]
- An overview on the histogenesis and morphogenesis of salivary gland neoplasms and evolving diagnostic approaches. Cancers (Basel). 2021;13:3910.
- [CrossRef] [PubMed] [Google Scholar]
- Salivary gland development: A template for regeneration. Semin Cell Dev Biol. 2014;25-6:52-60.
- [CrossRef] [PubMed] [Google Scholar]
- A case-control study of risk factors for salivary gland cancer in Canada. J Cancer Epidemiol. 2017;2017:4909214.
- [CrossRef] [PubMed] [Google Scholar]
- Histogenesis and morphogenesis of salivary gland neoplasms In: Surgical Pathology of the Salivary Glands. Philadelphia, PA: W.B. Saunders; 1991. p. :108.
- [Google Scholar]
- Surgical Pathology of the Salivary Glands Philadelphia, PA: Saunders; 1991. p. :108-421.
- [Google Scholar]
- Pathophysiology of myoepithelial cells in salivary glands. J Oral Maxillofac Pathol. 2016;20:480.
- [CrossRef] [PubMed] [Google Scholar]
- Histogenesis of salivary gland neoplasms. Ind J Can. 2013;50:361-6.
- [CrossRef] [PubMed] [Google Scholar]
- Pleomorphic adenoma: A systematic review. Int J Clin Pediatr Dent. 2020;13:284-7.
- [CrossRef] [PubMed] [Google Scholar]
- Warthin's tumour: A case report and review on pathogenesis and its histological subtypes. J Clin Diagn Res. 2014;8:37-40.
- [Google Scholar]
- Tumours of salivary glands In: WHO Classification of Tumours of the Head and Neck (4th ed). Lyon: IARC Press; 2017. p. :159-97.
- [Google Scholar]
- Acinic cell carcinoma of the parotid gland with four morphological features. Iran J Pathol. 2016;11:181-5.
- [Google Scholar]
- Learning from oncocytic tumors: Why choose inefficient mitochondria? Biochim Biophys Acta. 2011;1807:633-42.
- [CrossRef] [PubMed] [Google Scholar]
- The myoepithelial cell differentiation of mucoepidermoid carcinoma in a collagen gel-based coculture model. J Oral Pathol Med. 2004;33:237-42.
- [CrossRef] [PubMed] [Google Scholar]
- Epithelial myoepithelial carcinoma of the parotid gland. Med J Dr DY Patil Univ. 2015;8:772-4.
- [CrossRef] [Google Scholar]
- Histologic spectrum of polymorphous adenocarcinoma of the salivary gland harbor genetic alterations affecting PRKD genes. Modern Pathol. 2020;33:65-73.
- [CrossRef] [PubMed] [Google Scholar]
- Immunohistochemical characterization of basal cell adenomas of the salivary gland. Path Res Pract. 1991;187:145-56.
- [CrossRef] [PubMed] [Google Scholar]
- Immunohistochemical analysis of salivary gland tumors: Application for surgical pathology practice. Acta Histochem Cytochem. 2012;45:269-82.
- [CrossRef] [PubMed] [Google Scholar]
- A review: Immunological markers for malignant SGNs. J Oral Biol Craniofac Res. 2014;4:127-34.
- [CrossRef] [PubMed] [Google Scholar]







