Translate this page into:
Role of Solanum nigrum in the apoptotic and proliferative mechanism – Novel insights in therapeutic strategy of oral cancer

*Corresponding author: Thamizhchelvan Harikrishnan, Dean - Sri Ramachandra Dental college, Professor & Head, Department of Oral and Maxillofacial Pathology, Chennai, Tamil Nadu, India. hod.oralpathology@sriramachandra.edu.in
-
Received: ,
Accepted: ,
How to cite this article: Gnanasekar SS, Harikrishnan T, Ramalingam S, Jeyabalan S, Logeshwari. B. Role of Solanum Nigrum in the apoptotic and proliferative mechanism – Novel insights in therapeutic strategy of oral cancer. J Global Oral Health. 2024;7:57-62. doi: 10.25259/JGOH_6_2024
Abstract
Solanum nigrum (SN), referred to as black nightshade, is a member of the Solanaceae. It is composed of various alkaloids, glycoproteins, antioxidants, and flavonoids. It has abundant anticancer and antioxidant properties. Cancer is a global disease for which many therapies are still under research since there is no specific drug that could cure cancer to date. There are many types of cancer, which include breast, prostate, colorectal, lung, pancreatic, and oral cancer (OC). Among these, the sixth most prevalent type of cancer is OC. Treatment of cancer includes surgery, chemotherapy, and radiotherapy. There are many side effects associated with it. To prevent complications, phytotherapy will be a beneficial treatment option. This review will highlight the properties and components of SN and its various roles in different types of cancers. SN has several components in it of which each has a specific role and has several benefits. There are ongoing studies that explain the advantage of using this as a drug in different cancers; yet, this review highlights the pathway that has been involved in oral squamous cell carcinoma, and it could also be used as therapy.
Keywords
Solanum nigrum
Anticancer property
Oral cancer
Cell cycle
Anti-proliferative activity
Therapeutic strategy
INTRODUCTION
Solanum nigrum (SN) belongs to the family Solanaceae. It is a branching annual herb that grows at a height of 90 cm[1] also known as black nightshade, Manathakkalikeerai in Tamil, and Makoya in Hindi.[2] It is composed of alkaloids, steroidal saponins, glycoproteins, antioxidants, flavonoids, etc. It has abundant anticancer and antioxidant properties. The fruits of this plant are rich in solasodine. In ancient days, the leaves of this plant were traditionally used to treat mouth ulcers and tuberculosis.[3] This review will highlight the properties and mechanism of SN and its role in cancer.
The term cancer is a name derived from the Greek word “karkinos”, meaning carcinoma.[4] Breast cancer stands at the top with the highest global mortality rate, which has a prevalence rate of 15.2%. Among the different types, with an incidence of 14.7% worldwide, prostate cancer ranks as the second most frequent type of cancer. In 7.8% of cases, colorectal cancer is the third most frequent type of cancer. Pancreatic cancer accounts for the 12th most common cancer, with a hike in new cases by 3.3% and accounts for 8.3% of cancer deaths.[5]
The sixth most frequent malignancy worldwide is oral squamous cell carcinoma (OSCC) or oral cancer (OC), which is considered a serious health issue in developing countries.[6] About one-fourth of all cases worldwide are reported from India, where there are about 52,000 reported deaths and 77,000 new cases each year.[7] When compared to Western countries, the growing incidence of OC is the most significant public health concern. Concern about OC is particularly high in India, where approximately 70% of cases are reported to be advanced.[8] Squamous cell carcinoma of the lips, oral cavity, and oropharynx is typically the primary cause of OC.[9] The etiology of OC is multifactorial: smoking, tobacco chewing, and alcohol consumption. The treatment of OC is a multi-phased approach, which includes surgery, chemotherapy, and radiotherapy, which have many side effects. The acute side effects include oral mucositis, oral candidiasis or oral thrush, and xerostomia (dry mouth).[9] The long-term complications include trismus (difficulty in mouth opening), radiation caries, osteoradionecrosis, and necrosis. One of the major side effects of chemotherapy is that it acts on rapidly dividing tumor cells and normal cells,[10] Herbal drugs play a key role in saving normal cells. For their medical needs, most people on the planet still rely on herbal remedies using plants or herbs to treat diseases called phytotherapy.[11] Phytotherapy, also called phytomedicaments, is a therapeutic plant-based drug therapy associated with cancer.[12] The phototherapeutic medicine explained in this article is SN. This review will highlight the components, properties, and role of SN in various cancers.
Solanum nigrum contains rich natural components. There are a total of 188 phytoconstituents, which include steroidal components such as steroidal saponins, steroidal alkaloids, glycoproteins, polysaccharides, benzoic acids, and naturally occurring compounds.[13] Steroidal saponins are secondary metabolites that are more commonly found in plants and marine organisms. These are the major constituents present in SN. In the year 2006, a study was conducted, that identified six steroidal saponins, solanigrosides, and degalactotigonin from SN. These compounds were investigated for cytotoxicity on human tumor cell lines. They found that the component degalactotigonin was cytotoxic with inhibitory concentration (IC50) (0.25 ± 4.49 μM).[14]
Glycoalkaloids are abundantly present in fruits, stems, and leaves of the plant. The immature fruits of SN are rich in steroidal alkaloids (4.2%). In 2017, a study was conducted, and they isolated four steroidal components, namely, solanine, khasianine, and solamargine. Chemical techniques and spectroscopic data analysis were used to elicit these structures. They discovered that, with an IC50 of 3.85 ± 0.71 μM, the solanine component (solanine A) had the strongest inhibitory efficacy. Moreover, it showed notable cytotoxicity towards the human hepatoma (HepG2), primary adenocarcinoma of the colon (SW480), and human gastric carcinoma (MGC803) cell lines.[15] The polysaccharides have abundant antioxidant, antitumor, and hepatoprotective properties. The polysaccharides present in SN include vitamins, minerals, pigments, and amino acids.[16] The properties of SN are depicted in [Figure 1].
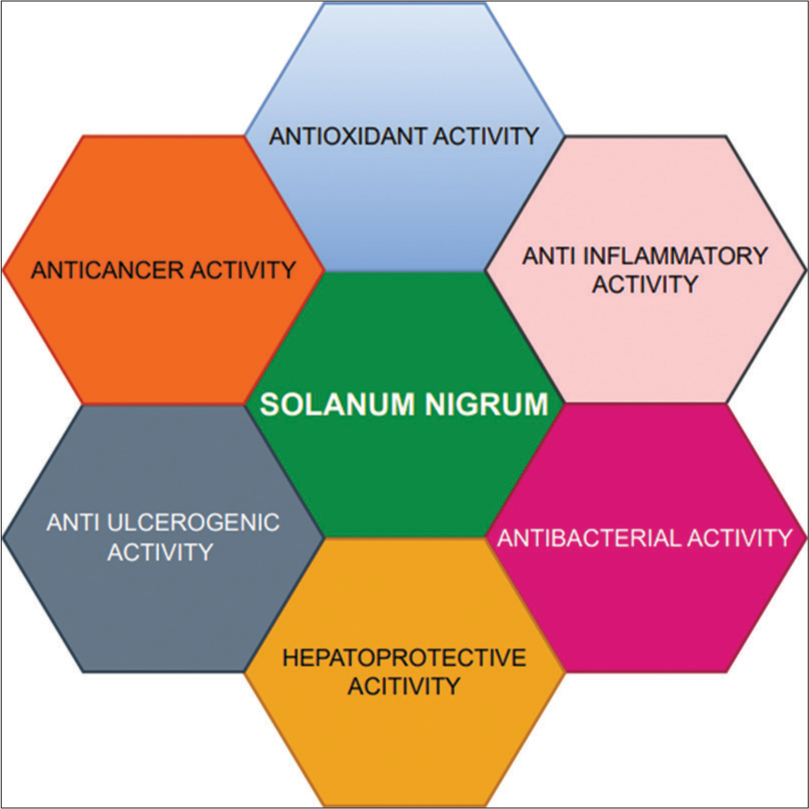
- Properties of Solanum nigrum (SN).
Cell cycle refers to the sequence of processes that occur in a cell that will eventually lead to its division and replication. There are three stages and five phases; the stages include the quiescent stage or the senescent stage, interphase, and mitosis. The phases include G0 (resting phase), G1 (Gap 1), S (Synthesis), Gap 2 (G2), and mitosis.[16] In the year 2020, a study was conducted to determine the effects of SN ethanolic extract on human breast cancer cell lines (MCF-7) in terms of cytotoxicity, cell cycle arrest, and apoptotic induction. They discovered that the extract stopped the tumor cells’ growth in the S phase and allowed them to proceed to the G2/M phase at half of the IC50. In addition, they discovered that solamargine, a component, demonstrated strong anticancer activity.[17] A study was carried out in 2022 to investigate the cytotoxic activity and cell cycle arrest of degalactotigonin (steroidal saponin) in triple-negative breast cancer cells (MDA MB231). They discovered that the component extract affected the cell accretion in the G2/M phase, which was concomitant with the cell cycle arrest.[18]
The anticancer efficacy of SN (Aqueous extracts of Solanum nigrum [AESN]) on breast cancer cell lines (MCF-7) was investigated in 2016. A 12-hour treatment with AESN resulted in the arrest of breast cancer cells in the G2/M phase. In addition, they discovered that ZEB1, N-cadherin, and vimentin were downregulated and that E-cadherin was elevated. Accordingly, they deduced that in breast cancer cell lines, these findings might prevent epithelial-mesenchymal transition (EMT).[19]
A study in 2017 examined the growth-inhibiting effects of the glycoalkaloid solamargine on non small cell lung cancer (NSCLC) cells. Solamargine was discovered to greatly raise the fraction of cells at the G0/G1 phase and prevent the proliferation of lung cancer cells. Thus, it was demonstrated that solamargine inhibited tumor cell growth.[20] The mechanism of cell death mediated by the chloroform fraction of the methanolic extract of SN on cervical cancer cell lines was investigated in research in 2017. The extract was observed to suppress the G1/S phase.[21] Hepatic carcinoma HepG2 and cervical cancer Hela cell line were the human cancer cell lines studied in 2021 to determine the in vitro cytotoxic potential of SN. The compound’s cytotoxic action was verified by high-performance liquid chromatography analysis. They discovered that the extracts of SN caused the animals to experience minute toxicity and cell cycle arrest in the G0 and G1 phases.[22]
In 2019, a study was conducted to determine the apoptotic activity of CM-319 (human chondroma cells) using the phytocomponent solamargine (steroidal glycoalkaloid). They found that solamargine controlled the proliferation of tumor cells and upregulated caspases 3, 8, and 9. Therefore, the results concluded that solamargine might be a novel therapeutic agent in treating chondroma.[23] The antiproliferative effect of SN ethyl acetate extracts on three cell lines – human colorectal adenocarcinoma (HT-29), ovarian cancer cell lines (A2780), and breast cancer cell lines (MCF-7) – was investigated in Sudan in 2014. The researchers discovered that SN ethyl acetate extracts suppressed the cell cycle in the S phase and caused early apoptosis. Therefore, the findings are consistent with the historic use of SN in several Sudanese regions for the treatment of cancer.[24]
In 2011, a study was conducted to study the effects of SN extracts high in polyphenols on PZ-HPV-7 human prostate cancer cell lines. They discovered that there was a dose-dependent arrest in the G2/M phase following treatment with SN extracts rich in polyphenols. Thus, they deduced that the extracts can accelerate apoptosis and specifically limit cellular proliferation in prostate cancer.[25] In 2018, a study was conducted in OC lines (SCC-4) to ascertain the anticancer properties of an AESN. In the cancer mechanism, cyclin B1 leads to the uncontrolled growth of tumor cells and is responsible for G2 to M phase transition. They found that cyclin-dependent kinase 1 and cyclin B1 were suppressed by treating AESN extracts. Further, AESN extracts promoted caspase 9 and 3, resulting in a mitochondrial apoptotic pathway. Thus, the findings indicated that by inhibiting mitochondrial activity, SN is useful in the treatment of OC.[26] The role of SN in cell cycle is depicted in Figure 2 and Table 1.
![Role of Solanum nigrum (SN) in cell cycle regression in various cancers. A phytocomponent called degalactotigonin inhibits the G1 phase in the cell cycle in pancreatic cancers. A glycoalkaloid called solamargine inhibits the G1 phase in breast and cervical cancers and also the G2 phase in breast cancer. SN inhibits the S phase in lung cancer.[17-22]](/content/7/2024/7/1/img/JGOH-7-057-g002.png)
- Role of Solanum nigrum (SN) in cell cycle regression in various cancers. A phytocomponent called degalactotigonin inhibits the G1 phase in the cell cycle in pancreatic cancers. A glycoalkaloid called solamargine inhibits the G1 phase in breast and cervical cancers and also the G2 phase in breast cancer. SN inhibits the S phase in lung cancer.[17-22]
| S. No. | Extract/phytocomponent used | Type of cancer | Cell cycle arrest | Property | Year of study | References |
|---|---|---|---|---|---|---|
| 1. | Ethanolic extract of SN-Solamargine | Breast cancer | S phase | Anticancer property | Churiyah et al. 2020 | [17] |
| 2. | Degalactotigonin extracts-Degalactotigonin | Breast cancer | G2/M phase | Anticancer property | Hamsa et al. 2022 | [18] |
| 3. | AESN | Breast cancer | G2/M phase | Anticancer, EMT inhibition | Lai et al. 2016 | [19] |
| 4. | Extract of SN Solamargine | Lung cancer | G0/G1 phase | Anticancer property | Chen et al. 2017 | [20] |
| 5. | Methanolic extracts of SN | Cervical cancer | G1/S phase | Apoptosis and anticancer property | Paul and Kundu, 2017 | [21] |
| 6. | Extracts of SN | Hepatic cancer, cervical cancer cell lines | G0 and G1 phase | In vitro cytotoxic property | Nawaz et al. 2021 | [22] |
| 7. | Extracts of solamargine | Chondroma | G1 phase Apoptosis: caspase 3,8,9 | Anticancer and apoptotic property | Liu et al.2019 | [23] |
| 8. | Ethyl acetate extracts of SN | Colorectal, ovarian and breast cancer | S phase | Apoptotic induction Anticancer property | Moglad et al. 2014 | [24] |
| 9. | Poly phenol-rich extracts of SN | Prostate cancer | G2/M phase | Anticancer property, inhibiting cellular proliferation | Akbar et al. 2012 | [25] |
| 10. | AESN | OSCC | CDK1 and Cyclin B1 | Anticancer property | Uen et al. 2018 | [26] |
SN: Solanum nigrum, AESN: Aqueous extracts of Solanum nigrum, OSCC: Oral squamous cell carcinoma, CDK1: Cyclin-dependant kinase 1, S: Synthesis phase, G1: Gap 1 phase, G2: Gap 2 phase, M: Mitotic phase, EMT: Epithelial-mesenchymal transition
Apoptosis (from root words meaning falling away from) is the programmed cell death in several important physiological and pathological processes.[27] In breast cancer, SN blocks the G2/M phase in the cell cycle and apoptosis pathway, which, in turn, activates caspase 3 and further inhibits EMT. The mature fruits of SN inhibit cell growth, which, in turn, promotes apoptosis in breast cancer cells.[20] Zinc oxide nanoparticles from SN were used in a study in 2021 to induce apoptosis in cervical cancer cell lines. Ultraviolet visual spectroscopy was used to create the nanoparticles. The researchers discovered that upregulating β catenin and elevating p53, caspase 3 and 9 levels. Therefore, they deduced that cervical cancer cell lines activation of apoptosis.[28] In 2017, a study was conducted in a panel of cervical cancer cell lines to ascertain the mechanism of the apoptosis-inducing pathway of the chloroform fraction of the methanolic extract of SN. They discovered that proapoptotic BAX, p53, and p21 expression rose while antiapoptotic BCL-2 expression reduced when cells were stopped in the G1/S phase. Thus, they concluded that when applied to cervical cancer cell lines, extracts from SN may have anticancer properties.[21]
In 2018 a study was demonstrated using solamargine, which is a phytocomponent present in SN. They found that the component solamargine significantly induced apoptosis by promoting Bax, caspase 3, and 7. Hence, they concluded that solamargine induced apoptosis and altered the mitochondrial membrane potential of cells.[29] In the year 2020, a study was conducted to determine the antitumor activity of solasonine on acute monocytic leukemia cell lines. They found that the component solasonine promoted the expression of Bax and cleaved caspase 3 and 9, inhibiting the anti-apoptotic protein BCL-2. Thus, they concluded that it has a positive effect on apoptosis in acute monocytic leukemia.[30]
In 2019, research was performed to investigate the component solamargine, a natural glycoalkaloid present in SN. They found that the level of cyclin D1 was significantly higher after treatment with solamargine. The expression of caspase 3, 8, and 9 and cyclin D1 was upregulated, and Ki -67 expression was downregulated. Therefore, the results suggested that solamargine might be a novel therapeutic agent in treating chondroma.[23] In 2017, a study was conducted to determine the anticancer activity of SN (AESN) extracts on OC. They found that AESN extracts markedly increased reactive oxygen species production. Furthermore, it also promoted caspase 3 and 9 activation and subsequent triggering of the mitotic apoptotic pathway. Hence, the results suggested that AESN extracts have the potential to be used as adjuvant chemotherapy in treating OC.[26] The role of SN in apoptosis is depicted in Figure 3 and Table 2.
![Potential target of Solanum nigrum (SN) in cancers- apoptotic pathway. α-solasonine inhibits BCL-2 and BCLXL in the apoptotic pathway. Phytocomponents called solanine, solamargine, and zinc nanoparticles with SN inhibit caspase 3 and caspase 7.[20,21,23,26,28-30] BCL: B cell lymphoma 2, FAS: Cell surface death receptor, FADD: Fas-associated death domain, Zn: Zinc, SMAC: Second mitochondria derived activator of caspases, APAF1: Apoptotic protease activating factor-1](/content/7/2024/7/1/img/JGOH-7-057-g003.png)
- Potential target of Solanum nigrum (SN) in cancers- apoptotic pathway. α-solasonine inhibits BCL-2 and BCLXL in the apoptotic pathway. Phytocomponents called solanine, solamargine, and zinc nanoparticles with SN inhibit caspase 3 and caspase 7.[20,21,23,26,28-30] BCL: B cell lymphoma 2, FAS: Cell surface death receptor, FADD: Fas-associated death domain, Zn: Zinc, SMAC: Second mitochondria derived activator of caspases, APAF1: Apoptotic protease activating factor-1
| S. No. | Extracts/photo component | Type of cancer | Apoptotic induction | Property | Year of Study | Reference |
|---|---|---|---|---|---|---|
| 1. | Mature fruits extract | Breast cancer | Caspase 3 Cell cycle: G2/M phase | Apoptotic induction and anticancer property | Chen et al.2017 | [20] |
| 2. | Zinc oxide nanoparticles of SN | Cervical cancer | P53, caspase 3,9 | Apoptotic induction | Thomas et al. 2021 |
[28] |
| 3. | Methanolic extract of SN | Cervical cancer | BAX, p53, p21 | Apoptotic induction | Paul and Kundu, 2017 |
[21] |
| 4. | Solamargine extract | Cholangiocarcinoma | BAX, caspase 3 and 7 | Apoptotic induction | Zhang et al. 2018 | [29] |
| 5. | Solasonine extract | Acute monocytic leukemia | BAX, caspase 3 and 9 BCL-2 (Inhibition) | Apoptotic induction | Zhang et al. 2020 | [30] |
| 6. | Solamargine extract | Chondroma | BCL-2 and BCL-XL Caspase 3,7 | Apoptotic induction | Liu et al. 2019 | [23] |
| 7. | AESN | OC | Caspase 3, 7 | Apoptotic induction (mitotic apoptotic pathway) | Uen et al. 2018 | [26] |
SN: Solanum nigrum, AESN: Aqueous extracts of Solanum nigrum, OC: Oral cancer, BCL-2: B cell lymphoma 2, Bax: BCL-2 associated protein
There are many studies conducted on SN in various types of cancer. However, the research for OC is still in the early stages. The limitations of SN include a lack of understanding in human trials. The toxicity levels of the plant also need to be assessed. Furthermore, studies have been conducted to assess the molecular mechanism of SN, but the results are not strong enough to develop a treatment modality. Therefore, further studies on SN are needed for approval before widespread use.
CONCLUSION AND FUTURE PERSPECTIVE
From this review, we can summarize that the pathways that are aberrantly active in other cancers are known to be active in the case of OC. Phytotherapy using solanum can also be used to treat OC since it could act on similar pathways to other various cancers. It could probably have the same effect of eradicating the tumor by initiating apoptosis and blocking the cell cycle through the same mechanism explained in this review. Numerous investigations have been carried out to assess SN anti-cancer potential in breast cancer, prostate cancer, hepatocellular carcinoma, and osteosarcoma. The use of SN could be a cost-effective drug and also a drug with not many complications in treating OC patients and patients undergoing chemotherapy. OC has the disadvantage of diagnosing it during the later stages, for which chemotherapy and radiotherapy are needed for a longer duration and can be reduced by the use of phytotherapy. The research on phytotherapy has only been in its early stages. Several challenges, such as the stage of diagnosis, metastatic tumor, nodal involvement, and recurrence of tumor, need to be considered. In the near future, it could be used as a major therapeutic strategy to eradicate OC with no complications for the patients.
Acknowledgments
I want to thank Shantha. S, Research assistant, Department of Oral Pathology and Microbiology, Sri Ramachandra Dental College, Chennai.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Antioxidant activity of the ethanolic extracts of leaves, stems and fruits of Solanum nigrum. Pharmacogn Commun. 2012;2:67-71.
- [CrossRef] [Google Scholar]
- An updated review on molecular genetics, phytochemistry, pharmacology and physiology of black nightshade (Solanum nigrum) Int J Pharm Sci Res. 2012;3:2956-77.
- [Google Scholar]
- Ancient Greek and Greco-Roman methods in modern surgical treatment of cancer. Ann Surg Oncol. 2010;17:665-7.
- [CrossRef] [PubMed] [Google Scholar]
- Available from: https://www.cancer.gov [Last accessed on 2024 Jan 17]
- Oral cancer diagnosis and perspectives in India. Sens Int. 2020;1:100046.
- [CrossRef] [PubMed] [Google Scholar]
- Role of Solanum nigrum novel insights in therapeutic strategy of oral cancer. J Oral Maxillofac Pathol. 2017;21:244-51.
- [CrossRef] [PubMed] [Google Scholar]
- Associations between oral hygiene habits, diet, tobacco and alcohol and risk of oral cancer: A case-control study from India. Cancer Epidemiol. 2017;51:7-14.
- [CrossRef] [PubMed] [Google Scholar]
- Review of the complications associated with treatment of oropharyngeal cancer: A guide for the dental practitioner. Quintessence Int. 2013;44:267-79.
- [Google Scholar]
- Polyherbal formulation: Concept of Ayurveda. Pharmacogn Rev. 2014;8:73-80.
- [CrossRef] [PubMed] [Google Scholar]
- Chapter 29: Phytochemicals: An immune booster against the pathogens In: Pati S, Sarkar T, Lahiri D, eds. Recent frontiers of phytochemicals. Netherlands: Elsevier; 2023. p. :501-9. Available from: https://www.sciencedirect.com/science/article/pii/B9780443191435000098 [Last accessed on 2024 Jan 17]
- [CrossRef] [Google Scholar]
- Phytotherapy and drugs: Can their interactions increase side effects in cancer patients? J Xenobiot. 2023;13:75-89.
- [CrossRef] [PubMed] [Google Scholar]
- Solanum nigrum Linn: An insight into current research on traditional uses, phytochemistry, and pharmacology. Front Pharmacol. 2022;13:918071.
- [CrossRef] [PubMed] [Google Scholar]
- Steroidal saponins from Solanum nigrum. J Nat Prod. 2006;69:1158-63.
- [CrossRef] [PubMed] [Google Scholar]
- Study on the effect of polysaccharides from Solanum nigrum Linne on cellular immune function in tumour-bearing mice. Afr J Tradit Complement Altern Med. 2013;10:41-6.
- [CrossRef] [PubMed] [Google Scholar]
- Cell cycle regulation by checkpoints. Methods Mol Biol. 2014;1170:29-40.
- [CrossRef] [PubMed] [Google Scholar]
- The cytotoxic, apoptotic induction, and cell cycle arrest activities of Solanum nigrum L. Ethanolic extract on MCF-7 human breast cancer cell. Asian Pac J Cancer Prev. 2020;21:3735-41.
- [CrossRef] [PubMed] [Google Scholar]
- Degalactotigonin induces cytotoxicity and cell cycle arrest in triple negative breast cancer cells (MDA MB 231) J Appl Pharm Sci. 2022;12:157-68.
- [CrossRef] [Google Scholar]
- Anti-cancer activity of Solanum nigrum (AESN) through suppression of mitochondrial function and epithelialmesenchymal transition (EMT) in breast cancer cells. Molecules. 2016;21:553.
- [CrossRef] [PubMed] [Google Scholar]
- Targeting EP4 downstream c-Jun through ERK1/2-mediated reduction of DNMT1 reveals novel mechanism of solamargine-inhibited growth of lung cancer cells. J Cell Mol Med. 2017;21:222-33.
- [CrossRef] [PubMed] [Google Scholar]
- Induction of apoptosis by fatty acid rich fraction of Solanum nigrum on cervical cancer cell lines. Int J Pharm Pharm Sci. 2017;9:199.
- [CrossRef] [Google Scholar]
- In vitro cytotoxic potential of Solanum nigrum against human cancer cell lines. Saudi J Biol Sci. 2021;28:4786-92.
- [CrossRef] [PubMed] [Google Scholar]
- Solamargine inhibits proliferation and promotes apoptosis of CM-319 human chordoma cells through suppression of notch pathway. Transl Cancer Res. 2019;8:509-19.
- [CrossRef] [PubMed] [Google Scholar]
- Ethyl acetate fraction of Solanum nigrum L: Cytotoxicity, induction of apoptosis, cell cycle in breast cancer cells, and gas chromatography-mass spectrometry analysis. Asian J Pharm. 2019;13:246-51.
- [Google Scholar]
- Selective cell cycle arrest and induction of apoptosis in human prostate cancer cells by a polyphenol-rich extract of Solanum nigrum. Int J Mol Med. 2012;29:277-84.
- [Google Scholar]
- Inhibition of aqueous extracts of Solanum nigrum (AESN) on oral cancer through regulation of mitochondrial fission. J Tradit Complement Med. 2018;8:220-5.
- [CrossRef] [PubMed] [Google Scholar]
- Programmed cell death (apoptosis) In: Molecular biology of the cell (4th ed). New York: Garland Science; 2002.
- [Google Scholar]
- Synthesis and characterization of zinc oxide nanoparticles of Solanum nigrum and its anticancer activity via the induction of apoptosis in cervical cancer. Biol Trace Elem Res. 2022;200:2684-97.
- [CrossRef] [PubMed] [Google Scholar]
- Solamargine derived from Solanum nigrum induces apoptosis of human cholangiocarcinoma QBC939 cells. Oncol Lett. 2018;15:6329-35.
- [CrossRef] [Google Scholar]
- Solasonine suppresses the proliferation of acute monocytic leukemia through the activation of the AMPK/FOXO3A axis. Front Oncol. 2021;10:614067.
- [CrossRef] [PubMed] [Google Scholar]







