Routine personal protective equipment in COVID-19 pandemic: What dentists need to know

*Corresponding author: Ankita Kar, Department of Head and Neck Oncology, Health Care Global Enterprises Pvt Ltd., Room No: 221, Tower 4, 2nd Fl, #8, P. Kalinga Rao Road, Sampan, Bengaluru - 560 027, Karnataka, India. drankita.k@hcgel.com
-
Received: ,
Accepted: ,
How to cite this article: Kar A, Bhaumik U, Kumar V, Shwetha V, Nagaraja S. Routine personal protective equipment in COVID-19 pandemic: What dentists need to know. J Global Oral Health 2021;4:102-6.
Abstract
The coronavirus pandemic of 2019 has increased the risk of occupational cross infections among dentists. Dental procedures are inherently risky in this scenario due to the need for close proximity with patients and generation of aerosols and splatters. Regulatory dental health bodies, including World Health Organization, Center for Disease Control, and American Dental Association, have devised guidelines for various forms Personal protective equipment for routine outpatient procedures during this pandemic and in the post-COVID-19 world. Stringent regulations are also advisable to conserve these resources at a time when the threat of COVID-19 is likely to persist indefinitely.
Keywords
COVID-19
Dentists
Personal protective equipment
N95 masks
Pandemic
INTRODUCTION
A cluster of pneumonia cases first reported in Wuhan, China, started in December 2019 has now rapidly transformed into a pandemic. An alert was raised by the Wuhan Municipal Health Commission and the Chinese Center for Disease Control and Prevention (China CDC). The World Health Organization (WHO) recognized as a pandemic in March 2020. At the beginning of 2020, the causative pathogen was identified as a novel coronavirus similar to severe acute respiratory syndrome (SARS) identified as SARS-COVID-2/COVID-19. Following this, genomic characterization and test method development ensued.[1]
Possible modes of transmission of COVID-19 include respiratory droplets, contact, fomites, and the feco-oral route.[2] During dental care, there are at least three possible sources of airborne contamination: Dental instrumentation, saliva and respiratory sources, and the operating site. Dental instrumentation contamination is the product of instrument-based organisms. The oral atmosphere is naturally moist with saliva that replenishes the fluid in the mouth continuously. Bacteria and viruses are grossly infected with the fluids in the mouth. A significant source of these species is dental plaque, both supragingivally and in the periodontal pocket. It should not be forgotten, however, that the oronasal pharynx is also part of the mouth. The mouth harbors bacteria and viruses from the nose, throat, and respiratory tract as part of this complex. These can involve numerous viruses and bacteria that are found in saliva and oral fluids that are pathogenic. Any dental procedure that has the potential to aerosolize saliva will cause airborne contamination with organisms from some or all of these sources.[3] Recent research suggests that COVID-19 can also be transmitted through aerosol-generating procedures, which are used by dentists.[4,5]
The recent pandemic spread of COVID-19 has posed substantial challenges for the field of medicine and dentistry. While routine dental care was suspended in countries experiencing COVID-19 disease during this period of the pandemic, the need for emergency care by teams provided with appropriate personal protective equipment (PPE) was prioritized. Emergency dental services were provided with advice on strict personal protection measures to reduce and avoid the production of droplets and aerosols, use of high-volume aspiration, and others.[6]
Dentists are at high risk of COVID-19 infection due to the close face-to-face contact and performing aerosol-generating procedures. PPE is an additional critical armamentarium used to protect dentists from COVID-19. The majority of dental procedures using mechanical instrumentation can create airborne particles from the place where the tool is used. The most visible aerosols are produced by dental handpieces, ultrasonic scalers, air polishers, and air abrasion units. Any of these tools eliminate from the operating site material that is aerosolized by the rotary instrument’s operation, by ultrasonic vibrations, or by the combined action of water sprays and compressed air. Usually, the water spray is the portion of the aerosol that is most visible to the naked eye and is noticed by the patient and dental personnel. It would be exceedingly difficult to conduct a qualitative and quantitative study of the composition of dental aerosols, and the composition of aerosols is likely to differ with each patient and the operating site. It is fair, however, to believe that the components of saliva, nasopharyngeal secretions, plaque, blood, components of the tooth, and any substance used in the dental process, such as air polishing abrasives and air abrasion, are all present in dental aerosols. In the past, research usually centred on the number of bacteria present in dental aerosols; several recent studies have analyzed the presence of blood components in dental aerosols.[7]
PPE IN DENTAL PRACTICE
During dental operations, asymptomatic COVID-19 carriers pose a high risk. Masks, caps, gowns, headcovers, and eye safety goggles or face masks are different kinds of PPE used by dentists to reduce transmission. These are recommended to protect the skin and mucosa from blood or secretions that are potentially infectious. For routine dental work, particulate respirators (e.g., N-95 masks or FFP2 standard masks set by the European Union) are recommended.[4,8]
WHEN DOES PPE HAVE TO BE WORN?
The New York Times, in their recent article, identified dentists to be at highest risk of getting COVID-19 from patients.[9,10]
The American Dental Association (ADA) developed guidelines on triaging patients and performing emergency dental procedures aiming at alleviating pain, preventing the progress of dental infections, and minimizing discomfort.[11]
EMERGENCY DENTAL PROCEDURES ARE MENTIONED BELOW
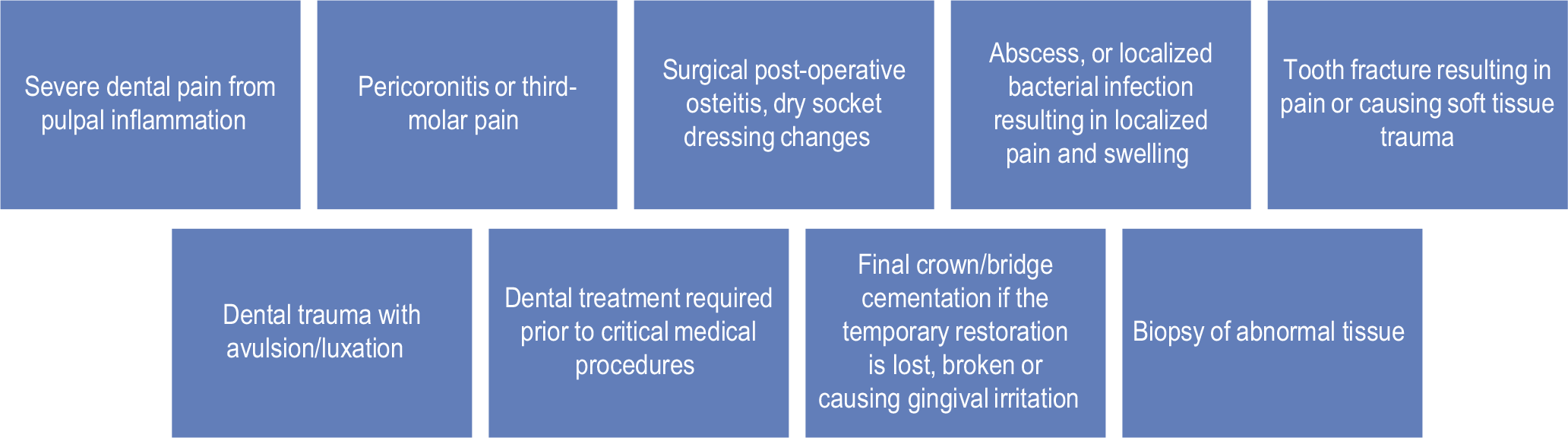
HOW ARE AEROSOLS PREVENTED BY USING PPE?
Splashes and droplets are inevitable during dental procedures, hence requires barriers to protect the eyes, nose, mouth, and upper respiratory tract of those exposed.
Aerosols are microscopic, lightweight particles with a neutral buoyant density that can remain in suspension in the air for extended time periods and travel long distances. These particles have a high likelihood of penetrating the respiratory tract up to the alveolar level. Aerosol particles can also be formed from the evaporation of a bigger droplet particle. These are termed “droplet nuclei” but behave like all other aerosols.[12]
Splatter
A mixture of air, water and/or solid substances (50 μm to several millimeters diameter). These also pose a significant health risk to the dental team due to the likely-hood of contamination with viruses. The COVID-19 virion measures around 120 nm (0.12 μm) and aerosol particle sizes range from 3–100 nm. Thus, the use of an FFP3 respirator effectively offers a filtration rate of 99% for virus, splatter, and aerosol particles measuring up to 0.6 μm.[13,14]
Gloves
Medical gloves are examples of PPE that is used to protect the wearer and/or the patient from the spread of infection or illness during dental procedures. The use of gloves is an essential part of an infection-control strategy. Disposable gloves include examination gloves, surgical gloves, and medical gloves for handling chemotherapy agents (chemotherapy gloves).
Gowns
While adequate PPE could provide protection from the exposures, all isolation gowns available on the market may not provide adequate protection to the wearer.
In the health-care setting, gowns are identified as the second-most-used piece of PPE, following gloves.[15,16] Association for the Advancement of Medical Instrumentation (AAMI) defines gowns as the protective apparel used to protect dentist and patients from the transfer of microorganisms and body fluids.[17]
Today, isolation gowns are produced from a wide variety of fabrics. They are generally classified as “disposable/single-use” or “reusable/multi-use”.
Disposable gowns are typically constructed of non-woven materials or in combination with materials such as plastic films. They can be produced using a variety of non-woven fiber-bonding technologies (thermal, chemical, or mechanical) to provide integrity and strength. The raw materials typically used for producing disposable gowns various forms of synthetic fibers (e.g., polypropylene, polyester, an polyethene). Fabrics can be engineered to achieve desired properties using particular fiber types, bonding processes, and fabric finishes.
Reusable gowns are laundered after each use. They are primarily made of cotton, polyester, or polyester/ cotton blends. These fabrics are tightly woven plain weave fabrics that are chemically finished and may be pressed through rollers to enhance the liquid barrier properties.[18]
N95 mask and surgical mask
The main advantage of an N95 respirator over a surgical mask is its filtration efficiency. It can achieve a tight seal, thus preventing air leakage around the edges. There are many models and sizes of N95 respirators. A successful “fit test” only qualifies to use the specific brand and size of respirator that is worn during that test (CDC and National Institute for Occupational Safety and Health 2020). Therefore, it should be apparent that “N95 respirators fitted to your face” mean that the dentist has been fit tested for an N95 respirator.[19]
Powered air-purifying respirator (PAPR) and N95
PAPR has been found to have a higher protective factor than N95 respirators. However, there is no conclusive evidence to show that PAPRs decrease the likelihood of viral transmission, especially the airborne transmission.[20]
Disadvantages of concurrent use include increased communication difficulties,[21] claustrophobic feelings,[22] and increased risk of infection from doffing the additional layers of PPE.[23] Proper doffing and cleaning of PAPR for re-use to prevent contamination and cross-infection is an essential step.[24] The assigned protection factor range is 25–1000 for PAPRs and 10 for N95 masks.[8]
Guidelines procedure
To minimize the risk of COVID-19 transmission in dental clinics, the ADA (2020b) provided three algorithms to assist dentists in making decisions on patient triage, evaluating for COVID-19, and minimizing risks for patients and staff during an emergency dental treatments. The goal was to minimize risks of transmission while allowing the provision of urgent care.[8]
The high demand of PPEs globally requires optimization of its use. There are guidelines recommended by the WHO and the CDC to maintain the supply in the wake of this increased need. Various strategies may be used to increase the re-use of PPEs during the COVID-19 pandemic that has modified the donning and doffing procedure.
Other methods of reducing transmission of aerosol
The use of a rubber dam would remove nearly all contamination resulting from saliva or blood during certain dental procedures. If it is possible to use a rubber dam, the only remaining source of airborne pollution is from the tooth that is being processed. This is restricted to the substance of the airborne tooth and any species found within the tooth itself. It is also difficult to use a rubber dam in such restorative procedures, such as subgingival restorations and the final stages of crown preparation.[3]
Two strategies are available to reduce airborne emissions arising from the operative site. One procedure involves the use of equipment which extracts the infected substance from the air in the treatment area after it has become airborne. The other is to kill the airborne pollutants before leaving the immediate area surrounding the operative site. High-quality particulate air, or HEPA, filtering and the use of ultraviolet or UV chambers in the ventilation system are the most commonly reported methods of eliminating airborne pollution from the air in the treatment space.[3]
High-efficiency particulate arrestor air filters (HEPA)
The physical removal of contaminants from the air is filtration and is a crucial step in achieving sufficient indoor air quality. It is also claimed that the air exchange rate in the operating room should be more than 15 times in 1 h (15 times × h [−1]) to maintain the air cleanliness in the operating room permanently. Various types of filtration, such as carbon, HEPA, or a mixture such as a carbon/HEPA filtration device are used by air purifiers. HEPA is suitable for air particles, while a filtration system is perfect for chemicals and odors in the air. As part of the Manhattan Project, HEPA filter was developed by the US Atomic Energy Commission in the 1940s to prevent the spread of airborne radioactive particles. The HEPA filtration technology was declassified and made available for commercial and residential use after World War II. The unit of filtration relies on the amount of square feet that should be cycled. According to the Institute of Environmental Sciences and Technology, 0.3 microns in size and larger, certified HEPA filters must catch at least 99.97% of pollutants. This ensures that only three can be allowed to escape for every 10,000 particles that move through the system. These are now used where exceptional air quality is needed, such as operating and isolating hospital rooms, the aerospace and pharmaceutical industries, and manufacturing plants for nuclear and computer chips around the world.[25]
PPE conservation and management
The WHO recommends strategies to maximize PPE use to protect health care workers (HCW) while minimizing the need for PPE in several ways, including the following:
Use of telemedicine for initial evaluation.
Only essential health care workers are allowed patient rooms.
Bundling activities to minimize the need for multiples entries.
Screening clinics will provide a dedicated area for evaluation of patients with potential COVID-19 and also allow for PPE conservation by staff.
CDC has devised a PPE burn rate calculator for health-care facilities to “plan and optimize the use of PPE for a response to COVID-19.” Whether surgical masks can be used instead of PPE in settings where risk of infection is minimal needs to be seen.
The Association for Professionals in Infection Control and Epidemiology recommend extended use of PPE over reuse.[5]
Limitations of PPE
The tolerability of HCW for PPE has not been studied in-depth, particularly during a pandemic where they have to be worn for long periods of time.
Communication problems (visual, auditory, or vocal), heat, pressure or pain, and dizziness or trouble concentrating can be potential reasons for discontinuation of the use of PPE. Problems found in the future by respondents that will need to be tackled include pain, breathing problems, and heat.
To ensure that HCW is properly secured, relaxed, and able to perform its work, it is important to understand the functional problems related to the nature of the PPE as well as the factors that affect the use. Future, research should be aimed at identifying factors that influence the comfort and tolerability of PPE and identifying changes that may affect comfort and tolerability.[26]
Amrita John et al. found that 14.9% of physicians did not have prior training in the use of PPE. Emphasis must be placed on proper doffing technique, and HCW must be reminded not to touch their eyes, face, or mucous membranes as these may lead to self-contamination.[24]
CONCLUSION
Protection of patients and dental staff during COVID-19 is a challenging aspect. Dental clinics and professionals are inadequately prepared to perform aerosol-generating procedures at the current, as they are not routinely fitted for the N95 respirators. SARS-CoV-2 is sensitive to many common disinfectants. This, coupled with prudent measures taken following the ADA and CDC guidance, can significantly reduce cross-transmission of the virus among dentists.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- The epidemiological characteristics of an outbreak of 2019 novel Coronavirus diseases (COVID-19)-China, 2020. China CDC Wkly. 2020;2:113-22.
- [CrossRef] [Google Scholar]
- Enteric involvement of Coronaviruses: Is faecal-oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol Hepatol. 2020;5:335-7.
- [CrossRef] [Google Scholar]
- Aerosols and splatter in dentistry: A brief review of the literature and infection control implications. J Am Dent Assoc. 2004;135:429-37.
- [CrossRef] [PubMed] [Google Scholar]
- Coronavirus disease 2019 (COVID-19): Emerging and future challenges for dental and oral medicine. J Dent Res. 2020;99:481-7.
- [CrossRef] [PubMed] [Google Scholar]
- Preparing for a COVID-19 pandemic: A review of operating room outbreak response measures in a large tertiary hospital in Singapore. Can J Anaesth. 2020;67:732-45.
- [CrossRef] [PubMed] [Google Scholar]
- Guidelines for dental care provision during the COVID-19 pandemic. Saudi Dent J. 2020;32:181-6.
- [CrossRef] [PubMed] [Google Scholar]
- The effectiveness of an aerosol reduction device for ultrasonic scalers. J Periodontol. 1997;68:45-9.
- [CrossRef] [PubMed] [Google Scholar]
- Dental care and oral health under the clouds of COVID-19. JDR Clin Trans Res. 2020;5:202-10.
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19 outbreak and its monetary implications for dental practices, hospitals and healthcare workers. Postgrad Med J. 2020;96:791-2.
- [CrossRef] [PubMed] [Google Scholar]
- Dental care during the Coronavirus disease 2019 (COVID-19) outbreak: Operatory considerations and clinical aspects. Quintessence Int. 2020;51:418-29.
- [CrossRef] [PubMed] [Google Scholar]
- Guidance on the use of respiratory and facial protection equipment. J Hosp Infect. 2013;85:170-82.
- [CrossRef] [PubMed] [Google Scholar]
- Dentistry and Coronavirus (COVID-19)-moral decision-making. Br Dent J. 2020;228:503-5.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluating the protection afforded by surgical masks against influenza bioaerosols: Gross protection of surgical masks compared to filtering facepiece respirators. Health Saf Exec. 2008;5:1.
- [Google Scholar]
- Taking cover: Single-use vs Reusable gowns and drapes. Infect Control Today. 2002;6:32-4.
- [Google Scholar]
- A bacteriologically occlusive clothing system for use in the operating room. J Bone Joint Surg Br Vol. 1983;65:502-6.
- [CrossRef] [PubMed] [Google Scholar]
- A review of isolation gowns in healthcare: Fabric and gown properties. J Eng Fiber Fabr. 2015;10:180-90.
- [CrossRef] [PubMed] [Google Scholar]
- Dental care and oral health under the clouds of COVID-19. JDR Clin Trans Res. 2020;5:202-10.
- [CrossRef] [PubMed] [Google Scholar]
- Practical recommendations for critical care and anesthesiology teams caring for novel Coronavirus (2019-nCoV) patients. Can J Anaesth. 2020;67:568-76.
- [CrossRef] [PubMed] [Google Scholar]
- Chemical protective clothing; a study into the ability of staff to perform lifesaving procedures. J Accid Emerg Med. 2000;17:115-8.
- [CrossRef] [PubMed] [Google Scholar]
- The effect on heart rate and facial skin temperature of wearing respiratory protection at work. Ann Occup Hyg. 2002;46:143-8.
- [Google Scholar]
- Safety recommendations for evaluation and surgery of the head and neck during the COVID-19 pandemic. JAMA Otolaryngol Head Neck Surg. 2020;146:579-84.
- [CrossRef] [PubMed] [Google Scholar]
- Role of high-efficiency particulate arrestor filters in control of air borne infections in dental clinics. SRM J Res Dent Sci. 2015;6:240-2.
- [CrossRef] [Google Scholar]
- Preventing Transmission of Pandemic Influenza and Other Viral Respiratory Diseases: Personal Protective Equipment for Healthcare Personnel: Update 2010 Washington, DC: National Academies Press; 2011.
- [Google Scholar]






