Sugar substitute: Key facts for their use – A review

*Corresponding author: Dr. Amrita Jaggi, Senior Lecturer, Department of Public Health Dentistry, SGT University, Gurugram, Punjab, India. amritakaur456@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Jaggi A, Marya CM, Oberoi SS, Nagpal R, Kataria S, Taneja P. Sugar substitute: Key facts for their use – A review. J Global Oral Health 2020;3(1):63-71.
Abstract
A simple, unidirectional schematic depicts the hypothesized pathways by which sugar-sweetened beverage consumption may lead to the development of chronic cardiovascular/cerebrovascular and metabolic diseases, chronic kidney disease, cancer, and gout. Sugar containing dietary foods could be replaced by the use of sugar substitutes available on the market today, both noncaloric and caloric, which have a low or even no cariogenic potential, sugar substitution is an important part of caries prevention and improving the overall health of an individual. The most common sugar substitutes used in Europe today are the caloric sweeteners xylitol, sorbitol, lycasin (hydrogenated starch hydrolysate), maltitol and mannitol and the non-caloric sweeteners accesultame-K, aspartame, cyclamate, and saccharin. They are currently replacing sugar in a wide range of products, such as sweeteners for coffee and tea, confectionery and chewing gum, medicines and soda pop. The need for a safe, palatable, non-nutritive, sweetening agent has prompted new approaches to the development of synthetic sweeteners. One interesting approach is based on the concept called “anatomical compartmentalization,” whereby the molecular weight of a sweet compound is increased to the point where no intestinal absorption occurs, thus eliminating systemic effects. Initial attempts at linking low molecular weight sweeteners to macromolecules have generally yielded products with unsatisfactory taste.
Keywords
Sugar substitutes
Sugars
Dental caries
Systemic diseases
Fermentable
INTRODUCTION
The term “sugar” refers to the common household foodstuff. When people describe how food tastes, they are actually talking about food flavor, and not just the basic tastes of sweet, sour, salty, and bitter. The range of flavor experiences also includes aroma, texture, and mouth “feel”- and, some would say, even the pleasantness of foods. The taste qualities are perceived through receptors located on the tongue and elsewhere in the oral cavity.[1] Of the four primary tastes (sour, salt, bitter, and sweet), sweetness is the only one that is pleasant at most concentrations, especially if one is hungry and often even if one is not. Most of us enjoy eating sweet-tasting foods, and some might almost have a psychological need for them. Sweetness is the taste that is strongly identified with affection and reward.[2]
A simple, unidirectional schematic depicts the hypothesized pathways by which sugar-sweetened beverage consumption may lead to the development of chronic cardiovascular/cerebrovascular and metabolic diseases, chronic kidney disease, cancer, and gout. These mechanisms have not been conclusively established by research studies, and several conflicting theories have been put forward. (1–8 from this article) According to the currently accepted concept, caries is truly a multifactorial disease. Interaction between three primary factors, namely, host tissue-the tooth, microflora with cariogenic potential, and suitable local substrate, i.e., diet is essential for initiation of the caries disease process (Keye, 1960). However, Newbrun (1982) added a fourth-factor “time” to the three above factors and the concept came to be known as “caries tetralogy.” Saliva is also considered an important factor as teeth are in continuous contact with saliva.[3]
Sugars are recognized as by far the most important dietary factor in the development of dental caries, and there is a clear understanding of the biology of the process of enamel dissolution induced by acid fermented products of sugars by the action of bacteria.[4] Fermentable sugars include both monosaccharides such as glucose and fructose, and disaccharides, such as sucrose, lactose, and maltose. Sugars in beverages may occur naturally (for example, lactose in milk), be released during processing (for example, fructose in 100% fruit juice) or be added during processing.[5]
Sugar containing dietary foods could be replaced by the use of sugar substitutes available on the market today, both noncaloric and caloric, which have a low or even no cariogenic potential, sugar substitution is an important part of caries prevention and improving the overall health of an individual.[6] The most common sugar substitutes used in Europe today are the caloric sweeteners xylitol, sorbitol, lycasin (hydrogenated starch hydrolysate), maltitol and mannitol and the non-caloric sweeteners acesulfame-K, aspartame, cyclamate, and saccharin. They all share the following characteristics: They can only be fermented by oral microorganisms to a very small extent or not at all, resulting in very low or no acid production. They are currently replacing sugar in a wide range of products, such as sweeteners for coffee and tea, confectionery and chewing gum, medicine, and soft drink. Invert sugar (hydrolyzed sucrose) is a commonly used sweetener for baby food. Xylitol has been claimed to have anticaries properties and thus to be superior to sorbitol, for example, a sugar substitute.[7]
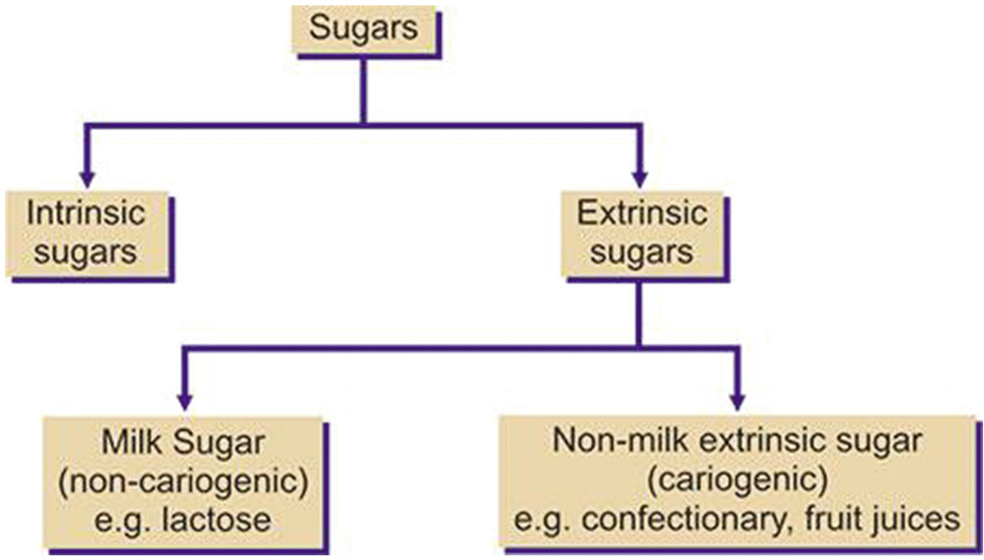
CLASSIFICATION OF SUGARS
According to the committee on medical aspects of food policy, sugars are classified as:
Intrinsic sugars
-
Extrinsic sugars.
Milk extrinsic sugars
Non-milk extrinsic sugars
USES OF SUGARS
Sugars have a number of functions in the preparation of foods, such as improving taste and texture.
Used in preservatives and jams, baking, canning and freezing, candy, general cooking, beverages, sugar also has some non-food uses:
In the fermentation process to make products containing alcohol (such as wine)
Setting of cement and glues
To help make certain types of detergents
In the textile industry for sizing and finishing fabrics
To make certain pharmaceuticals
Medical uses of sugar
Table sugar can be used to make oral rehydration solution (ORS), which can help prevent dehydration in children who have infantile diarrhea or vomiting in developing countries. The effective use of ORS saves millions of lives around the world each year. Although recipes for ORS vary from country to country, one widely used recipe is made up of 1 L of water, eight teaspoons sugar, and ½ teaspoon of salt.[2]
Sugar and fortification
Fortification of foods with micronutrients is generally recognized as the most cost-effective long-term strategy for eliminating micronutrient malnutrition. It is also socially acceptable, requires none or little change in food habits and characteristics, and provides a means for reaching the greatest percentage of the population requiring the micronutrients. Sugar is a safe and economical foodstuff that is accepted and consumed by populations at risk including those who are very poor. Hence, fortified sugar can play a critical role in fighting nutrient deficiency.[9]
Sugar is used as a vehicle for supplying Vitamin A in a number of Central American countries (Guatemala, Nicaragua, Honduras, and El Salvador) in Zambia and more recently in the Philippines. Pilot studies have also been conducted in other developing countries such as India and Vietnam. The consumption of fortified sugar has resulted in significant increases in Vitamin A intake and improvements in the Vitamin A status of a surveyed population in Guatemala. Vitamin A fortification can significantly reduce the risk of a permanent form of child blindness and mortality from severe infections in undernourished children.[9]
USES OF SUGAR SUBSTITUTES[10]
To assist in weight loss
Some people choose to limit their food energy intake by replacing high-energy sugar or corn syrup with other sweeteners having little or no food energy. This allows them to eat the same foods they normally would, while allowing them to lose weight and avoid other problems associated with excessive caloric intake.
| Natural (derived from plants) | Artificial |
|---|---|
| 1. Monellin 2. Dihydrochalcone 3. Miraculin 4. Xylitol 5. Thaumatins 6. Sorbitol 7. Stevioside 8. Isomalt 9. Erythritol |
1. Aspartame 2. Saccharin 3. Cyclamate 4. Acesulfame potassium 5. Alitame 6. Lactilol 7. High fructose corn syrup |
Dental care
Sugar substitutes are tooth-friendly, as they are not fermented by the microflora of the dental plaque. An example of a sweetener that can benefit dental health is xylitol. Xylitol works to prevent bacteria from adhering to the tooth surface, thus preventing plaque formation and eventually decay. The carbohydrates and sugars consumed usually adheres to the tooth enamel. Bacteria can feed on this food source, allowing them to quickly multiply. As the bacteria feed on the sugar, they convert it to acid waste that in turn decays the tooth structure. Xylitol cannot be fermented by these bacteria, so the bacteria have difficulty thriving, thus helping to prevent plaque formation.[11]
Diabetes mellitus
People with diabetes have difficulty regulating their blood sugar levels. By limiting their sugar intake with artificial sweeteners, they can enjoy a varied diet while closely controlling their sugar intake. Furthermore, some sugar substitutes do release energy but are metabolized more slowly, allowing blood sugar levels to remain more stable over time.[11]
Reactive hypoglycemia
Individuals with reactive hypoglycemia will produce an excess of insulin after quickly absorbing glucose into the bloodstream. This causes their blood glucose levels to fall below the amount needed for proper body and brain function. As a result, like diabetics, they must avoid intake of high-glycemic foods like white bread, and often choose artificial sweeteners as an alternative.[11]
Avoiding processed foods
Individuals may opt to substitute refined white sugar with less-processed sugars, such as fruit juice or maple syrup.[11]
Cost
Many sugar substitutes are cheaper than sugar. Alternative sweeteners are often low in cost because of their long shelf- life. This allows alternative sweeteners to be used in products that will not perish after a short period of time.[11]
Food industry
The food and beverage industry is increasingly replacing sugar or corn syrup with artificial sweeteners in a range of products traditionally containing sugar. Commonly consumed foods with sugar substitutes are diet sodas, cereals, and sugar-free desserts such as ice cream.[1]
SCIENCES OF SUGAR SUBSTITUTES
Natural sugar substitute derived from plants
Xylitol
Five-carbon sugar alcohol derived primarily from forest and agricultural materials. Xylitol was used initially by diabetics as its metabolism was considered to be insulin- independent.[12] It is naturally found in low concentrations in the fibers of many fruits and vegetables and can be extracted from various berries, oats, and mushrooms, as well as fibrous material such as corn husks and sugar cane bagasse, and birch.[10]
Uses – xylitol is a “tooth-friendly” non-fermentable sugar alcohol. Virtually all chewing gum sold in Finland is sweetened with xylitol. Specific brands of sugar-free gum containing xylitol include stride and trident. Finland in the 1970s found that a group chewing sucrose gum had 2.92 decayed, missing, or filled (dmf) teeth compared to 1.04 in the group chewing xylitol gums. In another study, researchers had mothers give xylitol gum to their children 3 months after delivery until they were 2 years old. The researchers found that children in the xylitol group had “a 70% reduction in cavities (dmf).”[13] Xylitol also has potential as a treatment for osteoporosis. A group of Finnish researchers has found that dietary xylitol prevents weakening of bones in laboratory rats, and actually improves bone density. Studies have shown that xylitol chewing gum can help prevent ear infections (acute otitis media); the act of chewing and swallowing assists with the disposal of earwax and clearing the middle ear, while the presence of xylitol prevents the growth of bacteria in the Eustachian tubes (auditory tubes or pharyngotympanic tubes) which connect the nose and ear.[13] A recent report suggests that consumption of xylitol may help control oral infections of candida yeast; in contrast, galactose, glucose, and sucrose may increase proliferation. Xylitol has an antimicrobial effect on bacteria such as Streptococcus pneumoniae and can be used to prevent ear infections (otitis media) in children, as found in a study by Tapiainen. For ear infections, xylitol can be administered as a nasal spray or by chewing gum, with chewing gum being the preferred method since chewing and swallowing helps massage the Eustachian tubes, which helps with lymph flow.[14]
Safety issues – during toxicity studies of xylitol by the Huntingdon Research Centre in England, it was found that mice ingesting diets with the highest xylitol content (10% and 20%) showed an increase in urinary bladder calculi, epithelial hyperplasia, and neoplasia of the bladder. Xylitol can be converted, through glycollate, to oxalate, which appears in the urine and can accumulate to form calcium oxalate crystals. The adverse effects observed in animal studies, such as pelvic nephrocalcinosis and bladder calculi, were induced by gross physiological and/or nutritional disturbance related to very high dose levels of the test compound.[15] Therefore xylitol at a dose high enough to form bladder stones may serve as a tumor promoter rather than a true carcinogen.
Sorbitol
Sorbitol is also known as glucitol, a sugar alcohol that the human body metabolizes slowly. It is obtained by the reduction of glucose, changing the aldehyde group to an additional hydroxyl group.[11] It was introduced in the diet of diabetics as early as 1929. In the 1970’s it also made a mark in the field of dentistry when various experiments were conducted to check the cariogenicity of sorbitol. Though Streptococcus mutans have enzymes to degrade sorbitol, the fermentation process is slow and hence very little drop in pH of the dental plaque was recorded after the ingestion of sorbitol as compared to the rapid drop following the use of sucrose.[16]
Uses – as a nutritive sweetener because it provides dietary energy: 2.6 kcal/g versus the average 4 kcal for carbohydrates. It often is used in diet foods (including diet drinks and ice cream), mints, cough syrups, and sugar-free chewing gum. It also occurs naturally in many stone fruits and berries from trees of the genus Sorbus.[17] Sorbitol can be used as a non- stimulant laxative through an oral suspension or enema. It works by drawing water into the large intestine, thereby stimulating bowel movements. Sorbitol is used in bacterial culture media to distinguish Escherichia coli 0154:H7 from most other strains of E. coli. Sorbitol often is used in mouth wash and toothpaste. Some transparent gels can be made only with sorbitol, as it has a refractive index sufficiently high for transparent formulations.
Adverse effects – sorbitol may aggravate irritable bowel syndrome, and similar gastrointestinal conditions, resulting in severe abdominal pain for those affected, even from small amounts ingested. Ingesting large amounts of sorbitol can lead to abdominal pain, gas, and mild to severe diarrhea. Sorbitol ingestion of 20 g/day as sugar-free gum has led to severe diarrhea leading to an unintended weight loss of 11 kg in a woman originally weighing 52 kg; another patient required hospitalization after habitually consuming 30 g/day.[16]
Thaumatin
It consists of 207 amino acids and has identical amino acid sequences except for five residues. These proteins are about 100,000 times sweeter than sucrose on a molar basis and 300 times sweeter on a weight basis.[6]
Uses – thaumatin is permitted as a sweetener in ice cream and sweets at levels up to 50 mg/kg and as a taste enhancer in soft drinks, desserts and dairy products at levels up to 0.5 mg/l and 5 mg/kg, respectively.[18] Thaumatin is effective at masking bitter notes often associated with pharmaceuticals or vitamins. Used at 20–400 ppm in pills and tablets, its long-lasting effect covers strongly bitter aftertastes and leaves a pleasant feeling in the mouth. Thaumatin can also be useful for masking astringency and off-flavors.[19]
Like other proteins, thaumatin is predicted to have a mainly beta structure, with a high content of beta-turns and little helix. Tobacco cells exposed to gradually increased salt concentrations develop a greatly increased tolerance to salt, due to the expression of osmotin, a member of the protein family. Wheat plants attacked by barley powdery mildew express a PR protein, which results in resistance against that infection. The similarity between this protein and other proteins to the maize alpha-amylase/trypsin inhibitor has suggested that proteins may act as some form of inhibitor.[11]
Stevioside
Stevioside and related compounds are responsible for the sweet taste of Stevia leaves.[20] Due to the high concentration of such sweet principles in leaves of Stevia plants, these are known as a honey leaf of sweet chrysanthemum.
Uses – stevia-based sweeteners fit well into this space, offering a combination of favorable attributes: High intensity and zero calories. There are two distinct markets: Specifically used for diabetic patients as a low calorie/dietetic segment and other as a nutritive sweetener which can be used as an alternative to marketed sugar (i.e., corn syrup, fructose, glucose, and sucrose).[21]
Recently, the stability of stevioside during different processing and storage conditions has been evaluated in tea and coffee beverages. Stevioside at elevated temperature for 1 h showed good stability up to 120°C. In aqueous solution, stevioside is remarkably stable in the pH range of 2–10. This knowledge seems to be essential for its effective application in hot coffee and tea beverages. It is anticipated that usage of stevioside in various food formulations, i.e., herbal tea, bakery, confectionery items, toothpaste, mouth refreshers, candies, and chewing gums may protect from the mentioned bacterium.[21]
Erythritol
It is a sugar alcohol (or polyol) that has been approved for use as a food additive in the United States[22] and throughout much of the world. Erythritol is 60–70% as sweet as sucrose (table sugar), yet it is almost noncaloric,[23] does not affect blood sugar, does not cause tooth decay, and is partially absorbed by the body, excreted in urine and feces. Under U.S. Food and Drug Administration (FDA) labeling requirements, it has a caloric value of 0.2 calories per gram (95% less than sugar and other carbohydrates), though nutritional labeling varies from country to country.
It is used in the beverage industry in the USA and Japan. It can also be used in food manufacture and chocolate, though usually, it needs to be mixed with other sweeteners to achieve the required texture and sweetness level. It is heat stable and can be used for cooking at home. It is a major ingredient of Truvia sweetener.[24]
Isomalt
A type of sugar alcohol, which is primarily used for its sugar- like physical properties. It has only a small impact on blood sugar levels and does not promote tooth decay.[25]
Uses – it does magic with flavors with its pleasant sweetness, enhances subtle, and fine flavors, such as peach, melon, passion fruit, and vanilla. The pure, natural taste of isomalt never overlays flavors but releases their full potential. It is very useful for confectioners and chefs for making showpieces as it is much more resistant to crystallization and more malleable than sugar.
Adverse effects – the reason that isomalt may prove upsetting to the stomach is because the body treats it as a dietary fiber instead of as a simple carbohydrate. Therefore, like most fibers, it can increase bowel movements and it passes through the bowel in virtually undigested form.[26]
Miraculin
It was sold as Miralin Miracle Fruit Drops (Miralin Co.), to be chewed before meals or snacks.[6] It has been suggested that the miraculin protein can change the structure of taste receptors on the cells of the tongue.[27]
Uses – it is effective in sweetening citrus fruits, berries, yogurt, and other sour foods but does not sweeten cereals, cocoa, coffee, etc. Miracle fruit is available as freeze-dried granules or in tablets – this form has a longer shelf life than fresh fruit. Tablets are made from compressed freeze-dried fruit which causes the texture to be clearly visible even in tablet form.[28] However, because manufacturers were unable to document their claims of efficacy in weight and reduced caloric intake, miraculin has been withdrawn from the market in the United States.[6]
Dihydrochalcone sweeteners
The sweetness of the dihydrochalcones is many times greater than sucrose; 75 however, unlike sugar, the dihydrochalcone sweetness is relatively slow in onset and is lingering. This after taste can be described as cooling like menthol, or licorice-like. Dihydrochalcones are stable over a broad range of temperatures, in aqueous solution, and in acids at normal temperature and therefore, potentially useful in fruits and carbonated beverage products.[7]
Uses – neohesperidin DH is permitted in a range of food products and beverages. Among these are desserts, yogurt, ice-cream, baked goods, jam, preserves, marmalade, soft drinks, sweets, mustard, and sauces. The permitted levels of use vary from 50 to 150 mg/kg depending on the food category.[18-46]
Monellin
It is a sweet protein, also known as serendipity berry, it was first reported as carbohydrate.[29] Monellin, thaumatin, and miraculin are the only taste-active proteins that have been currently characterized. Sweet protein monellin is approximately 3000 times sweeter than sugar. However, the protein has a slow onset of sweetness and a lingering aftertaste. The sweetness of monellin is pH and temperature- dependent.[30]
Uses – it can be useful for sweetening some foods and drinks, as it is a protein readily soluble in water due to its hydrophilic properties. However, it may have limited application because it denatures under high-temperature conditions, which makes it unsuitable for processed food. It may be relevant as non-carbohydrate tabletop sweetener, especially for individuals such as diabetics who must control their sugar intake.[31]
Artificial derived sugar substitute
Aspartame
It is about 200 times sweeter than sugar and is readily dissolvable in water. It has a sweet taste without the bitter chemical or metallic aftertaste reported in other artificial sweeteners. In addition to sweetening foods, aspartame is used to reduce calories, and intensify and extend fruit flavors.[17]
Aspartame is unstable under conditions of prolonged heating and is inappropriate for use in cooking and baking (Kroger et al., 2006). Aspartame also decomposes in liquids during prolonged storage. Aspartame-containing products are labeled to reflect these issues. Breakdown products include metabolic breakdown products (aspartic acid, phenylalanine, and methanol) and diketopiperazine. Breakdown results in loss of sweetness.[32]
Uses – enhances and extends flavors, does not promote tooth decay. Aspartame is an FDA approved, safe sweetening agent, and flavor enhancer that can be substituted for sugar in the diet.[16]
Saccharin
It is an aromatic organic compound used mainly in the form of its sodium salt. The consumption of saccharin has increased dramatically in many countries.[33]
Uses – about 70% of the total amount used in United States and Canada has been used in – diet soft drinks; 13% in dietetic foods (e.g., canned fruits, gelatin desserts, jams, and ice cream); 12% in products sold at retail for table use; and the remaining 5% in miscellaneous products such as mouthwashes, cosmetics, and medicinal preparations.
Safety issues – controversy regarding the safety of saccharin for human consumption was well underway. The possibility of a link between the use of saccharin and the risk of bladder cancer has been investigated; some studies found a weak positive association.[34] The status of saccharin as a possible over-the-counter product or the prescription item is still indeterminate.[6]
Cyclamates
At 1% dietary levels cyclamates produce a minimal laxative effect. Evidence indicates that this effect is attributable to the osmotic action of unabsorbed cyclamate in the intestine.[6]
It was banned because when embedded with cholesterol in a pellet in mouse bladders, or fed to rats at a dosage 50 times the daily maximum recommended for humans by the World Health Organization (WHO), induced bladder cancer. Experiments detected bladder cancer in 7–20 male rats treated. The animals received daily amounts equivalent to the total cyclamate contained in at least 500 eight- ounce bottle of a typical diet soft drink. The justification for such high dosage was to improve the chances of cancer detection. Solids (e.g., sodium stearate) can induce bladder cancer. Furthermore, a common rat bladder parasite also induces cancer. Recently a panel of scientists assembled by the National Cancer Institute reviewed more than 20 new animal-feeding studies and concluded that there was no firm evidence that cyclamates cause cancer, birth defects, or genetic damage. Cyclamate and saccharin may be tumor promoters rather than true carcinogens.[35]
Acesulfame K
It is a calorie-free sweetener that has been used in foods and beverages. The ingredient, which is 200 times sweeter than sugar, has been used in numerous foods in the United States since 1988. In the U.S., it is used in such products as candies, baked goods, frozen desserts, beverages, dessert mixes, and tabletop sweeteners. More than 90 studies have demonstrated the safety of acesulfame potassium. The ingredient is currently used in more than 4000 foods and beverages in about 90 countries around the world.[36]
Uses – it does not break down under high temperatures and so can be used in all processed foods and in cooking. It is literally thousands of products. Other than as a zero-calorie sweetener it has no other use. Acesulfame K is not harmful to teeth and suitable for diabetic patients. It is one of the cheapest sweeteners. Works very well with other sweeteners including aspartame and sucralose. It is heat stable and is suitable for cooking and in processed foods.
Safety issues – Acesulfame K shows no evidence of cariogenicity, mutagenicity, cytotoxicity, or teratogenicity. Pharmacokinetic tests indicate rapid and almost complete absorption. Excretion, preponderantly through the urine, is also complete because the compound does not accumulate in tissues or organs.[37]
Alitame
Like aspartame and neotame, alitame is a sweetener made from amino acids. Alitame is 2000 times sweeter than sugar. Only extremely small amounts would need to be used in a food or beverage, and the caloric contribution of alitame would, therefore, be negligible even though one of its components is metabolized.[38]
Uses – it is useful for diabetics and it is harmless to teeth. It is a totally artificial sweetener but it appears to be safe. Certainly, it is a better choice than Aspartame. Not well known or widely used, but has significant potential nonetheless. Unlike aspartame, phenylaniline is not a by-product of digestion and so it is suitable for people with the genetic disorder called phenylketonuria. It has quite a good taste and less aftertaste than aspartame. It is also more stable under heat though not as good as some sweeteners in this regard. So far, unlike aspartame, no side effects have been reported. Due to high cost, production has recently ceased. It is sold as Aclame.[18]
Lactitol
It has a good flavor with no aftertaste. It has two calories per gram (50% of sugar) but it has only 40% of the sweetness. Hence, on its own, it is of no special use as part of a calorie-controlled diet, sugar is better. It does, however, have a very low glycemic index and is consequently suitable for diabetics. It also has other unique properties which make it suitable as a replacement for sugar in certain applications.[38]
Uses – used in a variety of low food energy or low-fat foods. It has high heat stability, which it popular for baking. It is used in sugar-free candies, cookies (biscuits), chocolate, and ice cream. Lactitol also promotes colon health as a probiotic. Because of poor absorption, lactitol only has 2.4 calories (9 kJ) per gram, compared to four calories (17 kJ) per gram for typical carbohydrates. Lactitol is listed as an excipient in some prescription drugs, such as Adderall. Lactitol is a laxative and is used to prevent or treat constipation, for example, under the trade name importal. Lactitol in combination with ispaghula husk is an approved combination for idiopathic constipation as a laxative. Osmotic laxatives are often prescribed as first- line therapy in the management of constipation in children and adults.[39]
Safety issues – lactitol can cause cramping, flatulence, and diarrhea in some individuals. Because humans lack a suitable enzyme in the small intestine to digest it, a majority of lactitol reaches the large intestine, where it then becomes fermentable to gut microbes (prebiotic) and can pull water into the gut by osmosis, causing a laxative effect. Other than this, lactitol seems perfectly safe.[39]
High-fructose corn syrups (HFCS)
HFCS (also called glucose-fructose, isoglucose, and glucose-fructose syrup) 139,140 is a sweetener made from corn starch that has been processed by glucose isomerase to convert some of its glucose into fructose. HFCS, particularly one containing 55% of sucrose are being substituted for sucrose, invert sugar, and glucose syrups for use in processed foods and as a liquid table-top sweetener. The estimated use of HFCS for 1980 was 19 pounds per capita per year (8.6 kg). Soft drink manufacturers are the main users of HFCS, but HFCS use is increasing at a dramatic rate in other food industries such as baking, canning, processed foods, and dairy products.[6]
Uses – HFCS is found in various products ranging from cookies, sodas, crackers, and cereals, which makes it hard to avoid when consuming processed foods and drinks. It enhances other flavors because its sweetness is detected quickly and early by the taste buds, but does not linger, resulting in a clearer and crisper perception of other flavors. Maintains freshness and prolongs shelf life through improved moisture control and less microbial spoilage, resulting in firmer canned fruits and less freezer burn in frozen fruits. HFCS supplies are more reliable and predictable and its price is less volatile than that of sucrose; it is also slightly cheaper when costs are adjusted for equal sweetening power.[40]
PUBLIC HEALTH SIGNIFICANCE
However, sugar substitutes might be associated with some side effects. Literature shows that the use of artificial sweeteners can increase the risk of Type 2 diabetes mellitus. In 2014, Israeli research presented experimental evidence that artificial sweeteners may aggravate, rather than put a stop to, such as metabolic disorders like Type 2 diabetes.[41] Another research conducted in 2013 demonstrated that diets sweetened with either natural or artificial sugars are linked with an increase in Type 2 diabetes. However, more research is required. Artificial sweeteners can also cause an increase in weight and obesity. A study was carried out on 3682 individuals examining long-term relationships between artificially sweetened drinks and weight. The follow-up period was of 7–8 years in which weight was monitored. Results showed that those who consumed artificially sweetened drinks had a 47% higher increase in body mass index than those who did not. A similar hypothesis was proved by a study conducted at Texas University in 2005.[42]
Sugar-free chewing gums with sorbitol or xylitol as a substitute for sucrose have been available for a number of years and currently dominate the market. While the caries preventive effect has been primarily attributed to low or no acid production, the effect of chewing per se should not be ignored. All gums sugared and unsugared stimulate the flow of saliva, which leads to an increase in the buffering effect, as well as enhancement of remineralization and increased clearance of sugars from the mouth. It has been claimed that it would be difficult to obtain acceptance for a control chewing gum and the effect of chewing has therefore not been measured in previous studies. However, in the study by Machiulskiene et al.[43] a control gum with a non-caloric sweetener was used. The effect was close to that produced by adding xylitol to the chewing gum and points toward the need for further studies. A number of chewing gums have been developed that promote remineralization of enamel and some of them are now being marketed. These chewing gums contain sucrose substitutes in combination with a calcifying agent, such as calcium phosphate, phosphopeptide casein phosphopeptide-amorphous calcium phosphate complexes, or funoran.[44] More of these chewing gums with their added benefits will be developed and thus could assist in promoting dental health.
DISCUSSION
Many medicines have been found to have the side effect of producing xerostomia, and prolonged use of such drugs contributes to an increased risk of dental caries. Using non-cariogenic chewing gum to promote salivation would clearly be beneficial in these cases. The effect of sugar substitutes on changes in caries rates has been evaluated in several observational studies as well as clinical trials, with results consistently demonstrating a protective effect of xylitol on caries incidence. Sorbitol also was shown to decrease caries rates compared to controls; however, the reductions in caries rates were greatest when xylitol was the sugar substitute. Some limitations of previous studies include the lack of radiographs in caries diagnosis, high loss to follow-up, and potential confounding and bias due to the nature of long-term community intervention studies.[45] The criteria for causality – consistency, strength association, biologic plausibility, temporal sequence, and dose-response relationship – should be considered for evidence-based practice involving sugar substitutes. Sugar substitutes have marked their role in the dental industry also, as they are being frequently used in toothpastes, mouthwashes, mouth-fresheners, and chewing gums. The future prospects are bright for sugar substitutes accounting to the fast pace with which they are replacing natural sugars. However, the use should be within limits in view of their potential side effects. In addition, the dental professional should stay attuned to current information relative to alternative sweetener products that exist or are being developed and approved for dietary consumption by the FDA and be prepared to be a source of counseling for their patients and families as they relate to reducing the incidence of caries and weight gain.[46]
SUMMARY AND CONCLUSION
Most of the naturally occurring sugars are less sweet as compared to sucrose (sugar), while the chemically synthesized sugars (artificial sweeteners) have relative sweetness that is higher when compared to sucrose. Their consumption has been shown to cause mild to serious side effects ranging from nuisance headaches to potentially life- threatening cancer. Moreover, some of the sugar substitutes have remained in controversy due to their harmful fatal side effects and are been banned by FDA in some of the countries. Mainly aspartame has remained more controversial of all due to its potential carcinogenic and seizure promoting activity. Saccharin also carries a ban in some countries due to its hepatotoxicity and other side effects. Most of the artificial sweeteners are facing one or other controversy today. Scientists are divided in their views regarding the safety of these sweeteners. Xylitol is the most preferable sugar substitute while considering the dental aspects.
Recommendations
Food guide pyramid suggests a maximal intake of 25% of energy in the form of added sugars. It is advised to try using less sugar in recipes, limit to single serving size, use fresh fruit, using sugar substitutes to replace sugar in recipes
WHO recommends 10% of energy from added sugars, this recommendation is not based solely on scientific evidence but a range of epidemiological, economic, social, and political impacts on the prevention and control of non-communicable diseases
Individual with phenylketonurics should be cautioned for aspartame users as it may exaggerate the complication
Get most of your sugar from nature starchy foods instead of highly processed foods
Look for low sugar breakfast cereal with no more than 8 g of sugars/serving. Go easy on adding sugar to food.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Textual analysis of sugar industry influence on the world health organization's 2015 sugars intake guideline. Bull World Health Organ. 2016;94:566-73.
- [CrossRef] [PubMed] [Google Scholar]
- Understanding Dental Caries: Etiology and Mechanisms Basic and Clinical Aspects. Vol 1. Basel: Karger; 1985. p. :182-209.
- [Google Scholar]
- Role of sugar and sugar substitutes in dental caries: A review. ISRN Dent. 2013;2013:519421.
- [CrossRef] [PubMed] [Google Scholar]
- Preventing dental caries associated with sugar-sweetened beverages. J Am Dent Assoc. 2013;144:1148-52.
- [CrossRef] [PubMed] [Google Scholar]
- Sugar substitutes and non caloric sweetening agent In: Cariology (3rd ed). USA: Quintessence Publishing; 1989.
- [Google Scholar]
- Dietary factors in the prevention of dental caries: A systematic review. Acta Odontol Scand. 2003;61:331-40.
- [CrossRef] [PubMed] [Google Scholar]
- Uses of Sugars. Available from: http://www.wsro.org/aboutsugar/factsaboutsugar.aspx#section3. [Last accessed on 2018 Mar 11]
- [Google Scholar]
- The History of Artificial Sweeteners. Available from: http://www.trufax.org/research/f18.html. [Last accessed on 2018 Mar 14]
- [Google Scholar]
- Biochemical principles of the use of xylitol in medicine and nutrition with special consideration of dental aspects. Experientia Suppl. 1978;30:1-60.
- [CrossRef] [PubMed] [Google Scholar]
- Introduction of Xylitol. Available from: http://www.nstpainelimination.com/index.php?option=com_content&view=article&id=92&catid=36&Itemid=63&limitstart=2. [Last accessed on 2018 Mar 30]
- [Google Scholar]
- Dietary Xylitol Protects Against Osseal Changes in Experimental Osteoporosis. New York: Springer; 1998. p. :157-62.
- [CrossRef] [Google Scholar]
- Turku sugar studies XVIII. Incidence of dental caries in relation to 1-year consumption of xylitol chewing gum. Acta Odontol Scand. 1975;33:269-78.
- [CrossRef] [PubMed] [Google Scholar]
- Saccharin and other sweeteners: Mutagenic properties. Science. 1977;198:944-6.
- [CrossRef] [PubMed] [Google Scholar]
- Xylitol and oxalate. Metabollic studies in animals and man. Int J Vitam Nutr Res. 1985;55:1-152.
- [Google Scholar]
- Uses of Acesulfame-K. Available from: http://www.sugar-and-sweetenerguide.com/lactitol.html. [Last accessed on 2018 Sep 04]
- [Google Scholar]
- Nucleotide sequence of an osmotin-like cDNA induced in tomato during viroid infection. Plant Mol Biol. 1992;20:1199-202.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of the cariogenic potential of the intense natural sweeteners stevioside and rebaudioside A. Caries Res. 1992;26:363-6.
- [CrossRef] [PubMed] [Google Scholar]
- Determination of erythritol in fermented foods by high performance liquid chromatography. J. Food Hyg Soc Jpn. 1988;2:419-22.
- [CrossRef] [Google Scholar]
- Noncariogenicity of erythritol as a substrate. Caries Res. 1992;26:358-62.
- [CrossRef] [PubMed] [Google Scholar]
- Enthalpies of solution, partial molal heat capacities and apparent molal volumes of sugars and polyols in water. J Solution Chem. 1982;11:325-38.
- [Google Scholar]
- Introduction of Isomalt. Available from: http://www.beneonews.com/background_information/products/sugar_beet/isomalt/isomalt_short_brochure_engl.pdf. [Last accessed on 2018 Jun 23]
- [Google Scholar]
- Uses of Isomalt. Available from: https://www.liveto110.com/complete-list-of-artificialsweeteners. [Last accessed on 2018 Jun 30]
- [Google Scholar]
- Microbial production of sensory-active miraculin. Biochem Biophys Res Commun. 2007;360:407-11.
- [CrossRef] [PubMed] [Google Scholar]
- Functional expression of miraculin, a taste-modifying protein in Escherichia coli. J Biochem. 2009;145:445-50.
- [CrossRef] [PubMed] [Google Scholar]
- Introduction of Dihydrochalcone U.S. Department of Agriculture: Two Year Study of Neohesperidin Dihydrochalcone in Dogs. Washington DC: Western Regional Research Center; 1977.
- [Google Scholar]
- Redesigning a sweet protein: Increased stability and renaturability. Protein Eng. 1989;2:571-5.
- [CrossRef] [PubMed] [Google Scholar]
- Production of single chain recombinant monellin by high cell density culture of genetically engineered Candida utilis using limited feeding of sodium ions. J Chem Eng Jpn. 2002;35:654-9.
- [CrossRef] [Google Scholar]
- Sensory and Dietary Aspects in Aspartame Physiology and Biochemistry Book Florida: CRC Press Book; 1984.
- [Google Scholar]
- Diet and Dental Caries in the Prevention of Oral Disease (4th ed). Oxford: University of Oxford; 2003.
- [Google Scholar]
- Sucrose compared with artificial sweeteners: Different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr. 2002;76:721-9.
- [CrossRef] [PubMed] [Google Scholar]
- The sweetness-inducing effect of miraculin; behavioural and neurophysiological experiments in the rhesus monkey Macaca mulatta. J Physiol. 1983;337:221-40.
- [CrossRef] [PubMed] [Google Scholar]
- Microbiological aspects of some caloric sugar substitutes. Int Dent J. 1985;35:9-17.
- [Google Scholar]
- Diagnosis and treatment of chronic constipation a European perspective. Neurogastroenterol Motil. 2011;23:697-710.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis and management of functional constipation in children. JAAPA. 2013;26:21-4.
- [CrossRef] [PubMed] [Google Scholar]
- Adverse effects of xylitol in parental alimention. Metabolism. 1971;20:345-7.
- [CrossRef] [Google Scholar]
- Consumption of artificially and sugar-sweetened beverages and incident type 2 diabetes in the etude epidemiologique aupres des femmes de la mutuelle generale de l'education nationale-European prospective investigation into cancer and nutrition cohort. Am J Clin Nutr. 2013;97:517-23.
- [CrossRef] [PubMed] [Google Scholar]
- A role for sweet taste: Calorie predictive relations in energy regulation by rats. Behav Neurosci. 2008;122:161-73.
- [CrossRef] [PubMed] [Google Scholar]
- Caries preventive effect of sugar-substituted chewing gum. Commun Dent Oral Epidemiol. 2001;29:278-88.
- [CrossRef] [PubMed] [Google Scholar]
- Polyol chewing gums and caries rates in primary dentition: A 24-month cohort study. Caries Res. 1996;30:408-17.
- [CrossRef] [PubMed] [Google Scholar]
- Chewing gum facts and fiction: A review of gum-chewing and oral health. Crit Rev Oral Biol Med. 1999;10:405-19.
- [CrossRef] [PubMed] [Google Scholar]






