Platelet-rich fibrin: A promising regenerative material

*Corresponding author: Apoorva Vinayak Salve, Department of Periodontology, Swargiya Dadasaheb Kalmegh Smruti Dental College and Hospital, Nagpur, Maharashtra, India. apoorvasalve@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Uike S, Malik R, Salve AV. Platelet-rich fibrin: A promising regenerative material. J Global Oral Health 2023;6:55-8.
Abstract
Periodontal regeneration is a process involving biological events such as cell adhesion, migration, proliferation, and differentiation in an orchestrated sequence. It is well known that platelets play a key role in hemostasis and the wound healing process. Platelets were introduced as regenerative potential. Choukroun et al. (2001) first described platelet-rich fibrin (PRF). It is the second generation of platelet concentration. It consists of a natural fibrin matrix. It is autogenous fibrin made up of the patient’s blood free from anticoagulants. It is three-dimensional architecture consisting of the specific composition of leukocyte and platelet-rich fibrin biomaterials. Various technique has been used in the preparation of PRF but Choukroun’s PRF technique is the most common and easy technique developed in France. The patient’s blood was taken at around 5 ml which is collected in each of the two sterile vacutainer tubes with a 6 ml capacity without anticoagulant and centrifugation. PRF is used in various fields such as endodontics, oral and maxillofacial surgery, periodontics, and tissue engineering. It is more economical and easy preparation than other grafts. PRF should be handled properly before it shrinks and gets dehydrated. It is the most successful periodontal regenerative material.
Keywords
Platelet-rich fibrin
Regeneration
Platelets
INTRODUCTION
Periodontal disease is a multifactorial disease that is characterized by the loss of connective tissue attachment with the destruction of periodontal tissue leading to tooth loss. The goal of periodontal therapy is to eliminate the inflammatory process, prevent the progression of the disease, and regenerate lost periodontal tissues. Periodontal regeneration is a process involving biological events such as cell adhesion, migration, proliferation, and differentiation in an orchestrated sequence. The procedures include soft-tissue grafts, bone grafts, root biomodifications, guided tissue regeneration, and combinations of these procedures. Various regenerative materials have been used as autogenous bone grafts and allogenic bone grafts, but no one has a proven gold standard.[1]
Healing is a greater challenge in periodontal surgery. It is a complex process that leads to cell organization, chemical signals, and extracellular matrix for tissue repair. It is well known that platelets play a key role in hemostasis and the wound healing process. The rupture of blood vessels leads to fibrin formation, platelet aggregation, and the release of growth factors that play a role in wound healing.[2]
Platelets were introduced as regenerative potential. The platelets are used in concentration as platelet-rich plasma (PRP) and platelet-rich fibrin (PRF). Whitman et al. introduced PRP which enhances osteoprogenitor cells in host bone. PRP used should take precautions due to the risk of bovine thrombin, which may generate antibodies to factors V, XI, and thrombin leading to coagulopathies.[3]
Choukroun et al. first described PRF. It is the second generation of platelet concentration. It consists of a natural fibrin matrix. It is autogenous fibrin made up of patient blood free from anticoagulants. Its advantages are easy preparation with fewer chemical additives such as bovine thrombin and calcium chloride. Thus, this summarizes the relevant literature available on PRF as a promising regenerative biomaterial which focuses on the preparation of PRF, its clinical application, advantages and disadvantages.[4]
WHAT IS PRF?
PRF is a Choukroun’s PRF that was first developed in France and used in oral and maxillofacial surgery.[5] It has a three-dimensional architecture consisting of the specific composition of leukocyte and PRF biomaterials. PRF has various dense fibrin networks with leukocytes, cytokines, structural glycoproteins, and also growth factors such as transforming growth factor β1, platelet-derived growth factor, vascular endothelial growth factor, and glycoprotein such as thrombospondin-1 during P7 day. The concentration of leukocytes in the PRF scaffold plays a role in growth factor release, immune regulation, anti-infectious activities, and matrix remodeling during wound healing and slow polymerization takes place.[6,7]
According to the content of leukocyte and fibrin architecture, the platelets concentration was recently classified mainly in four available techniques: (1) Pure Platelet-Rich Plasma - Vivostat PRF, Anitua’s Plasma rich in growth factors (PRGF); PRGF-Endoret, or E-PRP all were in liquid suspension without leukocytes before activation. It can be activated and transformed into a gel form, (2) Leukocyte and Platelet-Rich Plasma, Curasan, Regen, Plateltex, SmartPReP, PCCS, and Magellan all were in liquid suspension with leukocytes before activation. It can be activated and transformed into a gel form, (3) Pure PRF - Fibrinet is a solid fibrin material without leukocytes, and (4) Leukocyte and PRF - Choukroun’s PRF; Advanced PRF, and injectable i-PRF, (Duo Process, Nice, France); L-PRF (Intra-Spin, IntraLock, Boca Raton, FL, USA); and Concentrated Growth Factors, a solid fibrin material with leukocytes.[8]
PROTOCOL FOR PREPARATION OF PRF
Various techniques have been used in the preparation of PRF but Choukroun’s PRF technique is the most common and easy technique developed in France. It is a second-generation platelet concentrate because of natural fibrin without any anticoagulants. The patient’s blood was taken at around 5 ml which is collected in each of the two sterile vacutainer tubes with a 6 ml capacity without anticoagulant. The vacutainer tubes are then placed in a centrifugal machine at 3000 revolutions/min for 10 min and immediately formed into three layers; upper straw-colored cellular plasma and the middle fraction containing the fibrin clot, the red lower fraction containing red blood cells. Mazor et al.[9] stated that after centrifuge, the fibrin clot gets transformed into a membrane formed when it is squeezed as shown in [Figures 1-3].

- Platelet-rich fibrin after centrifugation of blood.
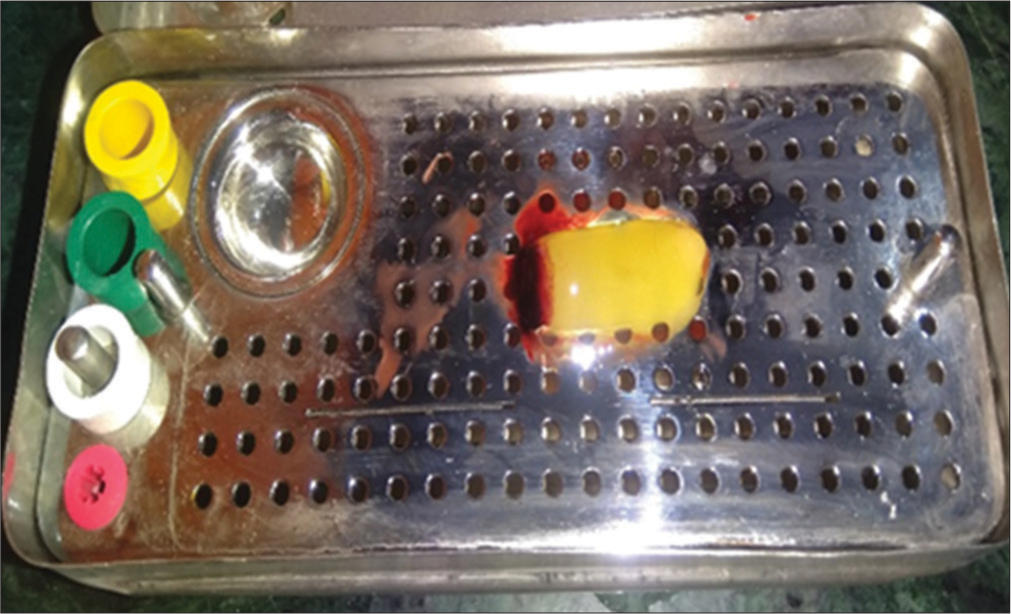
- Platelet-rich fibrin membrane box.
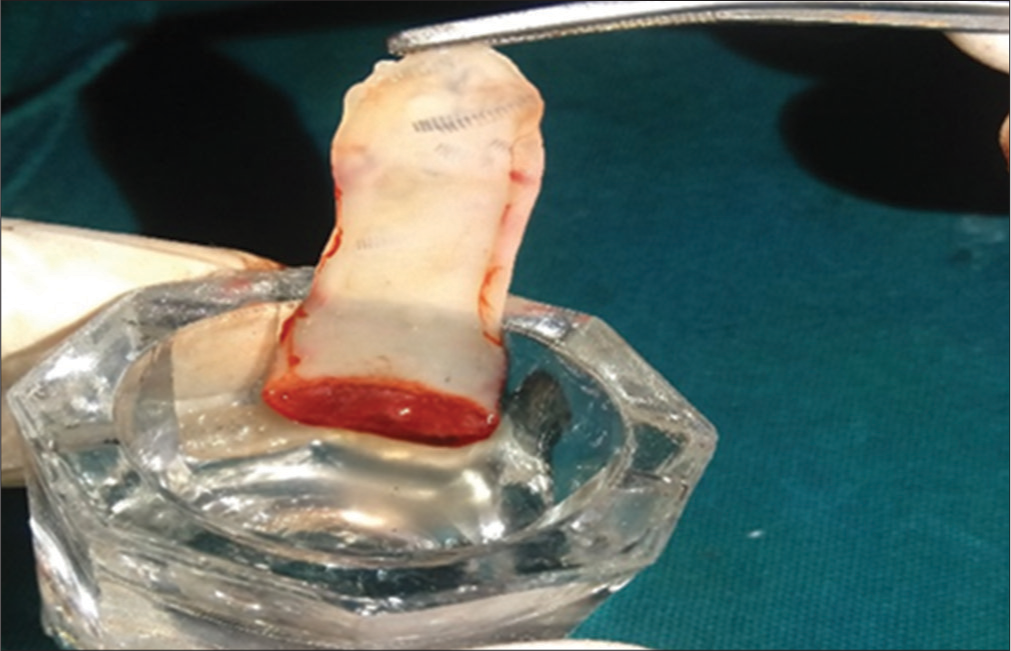
- Platelet-rich fibrin membrane after squeezing between gauze.
The upper part contains concentrated fibrinogen in the presence of thrombin and converts it into fibrin as shown in Figure 4. Blood starts converting into coagulation form in contact with glass surface due to lack of anticoagulant. When the silica surface contact, the clot polymerization process takes place. In this way, PRF is formed in glass-coated tubes which are easy to handle and quickly apply in the clinical area without any cytotoxicity.[10]
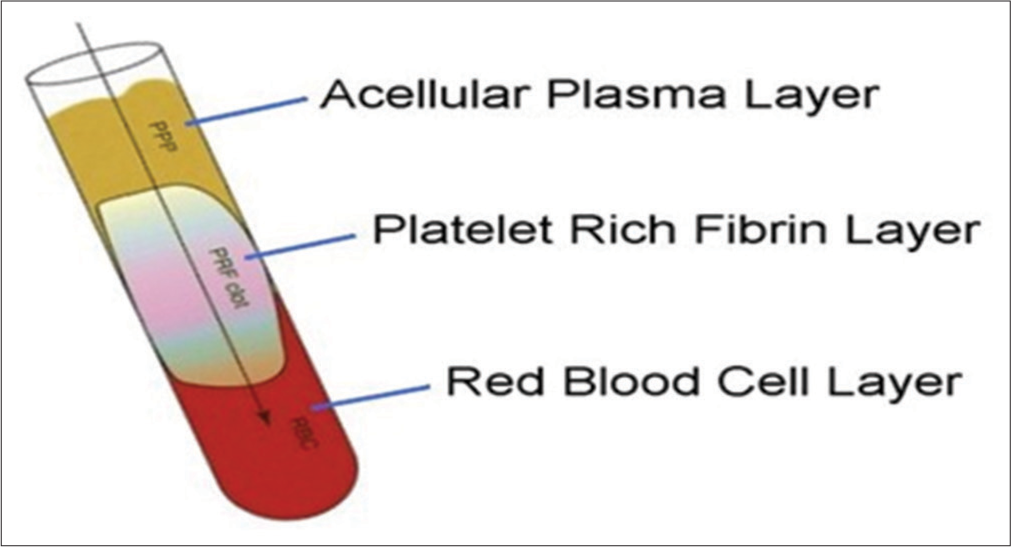
- PRF follows the preparation protocol and is divided into three parts: (1) Upper part – Acellular plasma layer, (2) Middle part – Platelets-rich fibrin, (3) Lower part – Red blood cell layer.
HANDLING AND PLACEMENT OF THE PRF
Platelet-rich fibrin (PRF) is then placed in the PRF box for the formation of membrane which is then cut and squeezed at the site according to its application in certain procedures. PRF can be mixed with other regenerative materials based on its requirement like bone graft or it can be used as a membrane to cover the graft placed.
CLINICAL APPLICATION
Endodontic applications
In the treatment of open apex
For regeneration of pulp-dentin complex
In combination with MTA to create root end barriers in apexification procedures to prevent extrusion of material
In regenerative pulpotomy.
Oral & maxillofacial surgery applications
Filling material in avulsion sockets and bony defects
Bone augmentation in sinus lifts for posterior maxilla augmentation for implants.
Periodontology applications
Ridge preservation-guided bone regeneration
For the treatment of intrabony defects, treatment of gingival recession, and treatment of GTR and periapical lesion
Thorat et al. investigated the clinical and radiological effectiveness of PRF in intrabony defects in chronic periodontitis. The result was observed to be more reduction in probing pocket depth, gain in clinical attachment level, and greater bone fills at the tested site with PRF and open flap debridement alone in control sites.[11]
Parikh et al. conducted a study to evaluate the success of the use of PRF in combination with pouch and tunnel technique for multiple gingival recession with a 6-month follow-up.[12]
Ustaoğlu et al. conducted a study to assess the adjunctive use of t-PRF in intrabony defects with open flap debridement in comparison with guided tissue regeneration as a gold standard and OFD alone as a control. They concluded that t-PRF may give similar successful results as GTR in the treatment of IBDs with endoperio lesions.[13]
Chenchev et al. conducted, a study to evaluate the stability of dental implants placed after socket preservation with allograft or PRF as a sole grafting material and conclude that formation of new bone cells around the implant surface increases the biological stability of the dental implant.[14]
PRF use as scaffolds in tissue engineering have been investigated by many researchers. Kang et al. conducted an in vitro study stating PRF as a bioscaffold and reservoir of growth factors for tissue regeneration.[15]
ADVANTAGES OF PRF
It is an easily prepared and simple technique with centrifugation
It is a purely autogenous blood sample
It does not require any additional chemical composition such as thrombin and without any immunological reactions
It can be used with a combination of bone grafts
It will rapidly be healed
It is economical and quickest option other than bone grafts
It is used as a membrane which reduces patients’ discomfort.[16]
DISADVANTAGES OF PRF
PRF success depends on mainly handling, related to blood collection time, and its transfer to the centrifuge machine
Use of glass-coated tubes for cloth polymerization
The clinician should be well experienced in the manipulation of PRF
PRF should be used immediately before it shrinks which dehydrates the structural integrity and decreases the growth factor content in PRF8.
CONCLUSION
PRF is a simple technique. It is the most successful regeneration of periodontal tissues. In the future, PRF and its applications in the field of soft tissue and bone regeneration have enormous therapeutic implications, but the role in dentistry may need more studies of randomized trials and standardized PRF processes that will enhance therapeutic outcomes.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- The potential role of growth and differentiation factors in periodontal regeneration. J Periodontal. 1996;67:545-53.
- [Google Scholar]
- Platelet gel: An autologous alternative to fibrin glue with applications in oral and maxillofacial surgery. J Oral Maxillofac Surg. 1997;55:1294-9.
- [CrossRef] [PubMed] [Google Scholar]
- Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part IV: Clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e56-60.
- [CrossRef] [PubMed] [Google Scholar]
- Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part III: Leucocyte activation: A new feature for platelets concentrates? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e51-5.
- [CrossRef] [PubMed] [Google Scholar]
- Antimicrobial activity of platelet-leukocyte gel against Staphylococcus aureus. J Orthop Res. 2008;26:404-10.
- [CrossRef] [PubMed] [Google Scholar]
- Classification of platelet concentrates: From pure platelet-rich plasma (P-PRP) to leucocyte-and-platelet-rich fibrin (L-PRF) Trends Biotechnol. 2009;27:158-67.
- [CrossRef] [PubMed] [Google Scholar]
- Sinus floor augmentation with simultaneous implant placement using Choukroun's platelet-rich fibrin as the sole grafting material: A radiologic and histologic study at 6 months. J Periodontol. 2009;80:2056-64.
- [CrossRef] [PubMed] [Google Scholar]
- Cytotoxicity analyses of Choukroun's platelet-rich fibrin (PRF) on a wide range of human cells: The answer to a commercial controversy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:587-93.
- [CrossRef] [Google Scholar]
- Clinical effect of autologous platelet-rich fibrin in the treatment of intra-bony defects: A controlled clinical trial. J Clin Periodont. 2011;38:925-32.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical evaluation of autologous platelet-rich fibrin in combination with pouch and tunnel technique for the treatment of multiple adjacent gingival recession defects-a case report. World J Adv Sci Res. 2019;2:81-91.
- [Google Scholar]
- Comparison of GTR, T-PRF and open-flap debridement in the treatment of intrabony defects with endo-perio lesions: A randomized controlled trial. Med Oral Patol Oral Cir Bucal. 2020;25:e117-23.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of primary and secondary stability of dental implants placed after socket preservation with allograft or PRF-a randomized controlled clinical trial. Clin Oral Implants Res. 2019;30:453.
- [CrossRef] [Google Scholar]
- Platelet-rich fibrin is a bioscaffold and reservoir of growth factors for tissue regeneration. Tissue Eng Part A. 2011;17:349-59.
- [CrossRef] [PubMed] [Google Scholar]
- Current knowledge and perspectives for the use of platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in oral and maxillofacial surgery part 2: Bone graft, implant and reconstructive surgery. Curr Pharm Biotechnol. 2012;13:1231-56.
- [CrossRef] [PubMed] [Google Scholar]







