The role of pre-surgical INR testing in dental patients with liver diseases

*Corresponding author: Behzad Mostoufi, Department of Oral and Maxillofacial Surgery, University of Maryland, Baltimore 21201, Maryland, United States. bmostoufi@umaryland.edu
-
Received: ,
Accepted: ,
How to cite this article: Mostoufi B, Clark A, Wilken N, Sands J, Meiller TF, Ord R. The role of pre-surgical INR testing in dental patients with liver diseases. J Global Oral Health 2020;3(2):89-93.
Abstract
Objectives:
The aim of this study was to evaluate the validity of international normalized ratio (INR) in patients with liver diseases or abnormal liver function tests as related to bleeding risk in dental procedures.
Materials and Methods:
From July 2008 to January 2019, the INR of 187 patients with liver diseases who underwent oral surgical procedures at the Department of Oral and Maxillofacial Surgery Clinic, University of Maryland School of Dentistry was collected and compared with normal value. Patients who were taking anticoagulants were excluded from the data pool.
Results:
The compiled INR for the 187 patients (M/F = 122/65) with mean age of 47 years (range: 22–77) was 1.126, with a median and mode of 1.1. The standard deviation was 0.17. The range for the INR values was 0.7 (n = 1) to 1.7 (n = 1).
Conclusion:
In the present study, there was no significant difference between the INR of patients with liver diseases or abnormal liver function tests and normal INR value. This supports the belief that pre-operative INR testing is not a dependable marker to assess bleeding risk in patients with chronic liver diseases who are not taking Vitamin K antagonist anticoagulants.
Keywords
Liver diseases
Liver failure
Bleeding risk
Prothrombin time
International normalized ratio
INTRODUCTION
In today’s practice, it is not uncommon to encounter patients with liver diseases in dental clinics for routine care or for dental clearance before liver transplant surgery. As health-care providers, it is paramount that we understand the risks associated with treating this patient population as well as formulating guidelines to help provide treatment safely.
Liver diseases have been recognized as having underlying coagulopathy disorders as the liver is responsible for creating majority of the clotting factors required for body to maintain normal hemostasis.[1] Thus, persistent bleeding is a common concern in patients with chronic liver disorders that require surgical dental procedures. Liver disease has many forms, but is most commonly attributed to chronic infections such as hepatitis B virus (HBV), hepatitis C virus (HCV), and cirrhosis.[1-3] According to the 2016 Centers for Disease Control and Prevention viral Hepatitis Surveillance Report, 850,000–2.2 million persons are estimated to be infected with HBV and 2.7–3.9 million individuals are chronically infected with HCV in the United States.[4,5] Acute illness can be characterized by nausea, malaise, abdominal pain, and jaundice. However, majority of acute infections are asymptomatic or only cause mild symptoms. Therefore, many individuals with HBV or HCV are unaware that they are carriers resulting in silent infections until developing cirrhosis, end-stage liver disease, or hepatocellular carcinoma.[5] Other causes of liver diseases include alcohol abuse, drug abuse, and biliary obstruction[4] [Table 1].
| Infection | Immune system abnormality | Genetics | Cancer and other growths | Other |
|---|---|---|---|---|
| Hepatitis A | Autoimmune hepatitis | Hemochromatosis | Liver cancer | Chronic alcohol abuse |
| Hepatitis B | Primary biliary cirrhosis | Hyperoxaluria and oxalosis | Bile duct cancer | Non-alcoholic fatty liver disease |
| Hepatitis C | Primary sclerosing cholangitis | Wilson’s disease | Liver adenoma | Drug induced |
The current practice is to have access to the patient’s recent laboratory studies to predict bleeding complications.[6] Standard laboratory workup includes obtaining a complete blood count, liver function tests, and coagulation panel including bleeding time, prothrombin time (PT)/international normalized ratio (INR), PT test, and platelet count.[6] However, coagulation test may only have limited predictive value for bleeding tendency.[1] To date, there is no defined protocol for clinicians to follow regarding treatment and evaluation of the pre-transplant liver failure patient population.[7]
Liver failure is characterized by defective hepatic synthesis of clotting factors and thrombocytopenia.[1] The liver is responsible for synthesizing 11 of the 13 blood coagulation factors, including fibrinogen (I), prothrombin (II), and factor V, VII, IX, X, XII, and XIII. In addition to procoagulation factors, anticoagulant factors such as anti-thrombin, protein C, and protein S are also synthesized by the liver2. In 1935, A.J. Quick developed the PT to investigate the coagulopathy associated with obstructive jaundice.[8] The test is responsive to detecting coagulation factors X, VII, V, fibrinogen (II), and prothrombin (I).[9] From there, the INR was derived to achieve standardization and calibration when comparing PTs from different laboratories. Since then, INR has been universally accepted as the method for monitoring bleeding risk for patients receiving Vitamin K antagonist (VKA).[9] However, over recent years, INR has been used to assess coagulopathies in patients with chronic liver diseases and to construct the model for end-stage liver disease score, which prioritizes patients for liver transplant.[8]
MATERIALS AND METHODS
For this study, formal application for the Institutional Review Board (IRB) review was made and University of Maryland, IRB determined that the study protocol to be exempt, a waiver of HIPAA authorization was also approved. Patients who were referred to the Department of Oral and Maxillofacial Surgery clinic at the University of Maryland School of Dentistry for oral surgical procedures from July 2008 to January 2019 were considered for this study. Patients with diagnosis of hepatitis B, hepatitis C, liver cirrhosis, excessive alcohol drinking habit, and/or abnormal liver function test who had INR value on file were selected. INR for these patients was not requested by treating surgeons, rather obtained by referring dentists or managing/consulting physicians as a routine laboratory test protocol to predict bleeding tendency before invasive dental procedures and was available to the surgeons at the time of treatment. Procedures for which patients were referred to the clinic included dentoalveolar surgeries, incisional and excisional soft/hard tissue biopsies, and pre-prosthetic surgeries under local anesthetic with or without sedation. None of the patients had diagnosis of liver or renal failure and clinical signs and symptoms of liver diseases were absent. Patients on any anti-coagulant therapy such as warfarin were excluded from the study. A total of 187 patients met the inclusion/exclusion criteria, and their INR values were analyzed and compared to normal. The accepted reference range for INR is <1.3; however, the normal range is highly variable and dependent on the laboratory performing the test.[10] In this study, normal INR value was populated from current standard normal range used by laboratories and medical centers across the USA and found to be 0.8–1.2.[10] The INR results were evaluated for range, mean, median, and mode, and a standard deviation was calculated. Values were plotted on a simple bar graph, and statistical significance calculated using a Z score and t-test. Confidence interval was also calculated with confidence level of 95%.
RESULTS
A total of 187 patients (122 M and 65 F) with a mean age of 47 years (range 22–77) had INR values on the record that were compiled and plotted [Figure 1]. The average INR for the surveyed population was 1.126, with a median and mode of 1.1. The standard deviation was 0.17. The range for the INR values covered 0.7 (n = 1) to 1.7 (n = 1), [Figure 2] we considered statistical significance to be P < 0.05. A Z score was calculated comparing the mean to the expected normal INR and using standard deviation, P value with one-tailed hypothesis was 0.33. Therefore, the difference between the INR values obtained from the study subjects and normal INR was not statistically significant [Table 2].
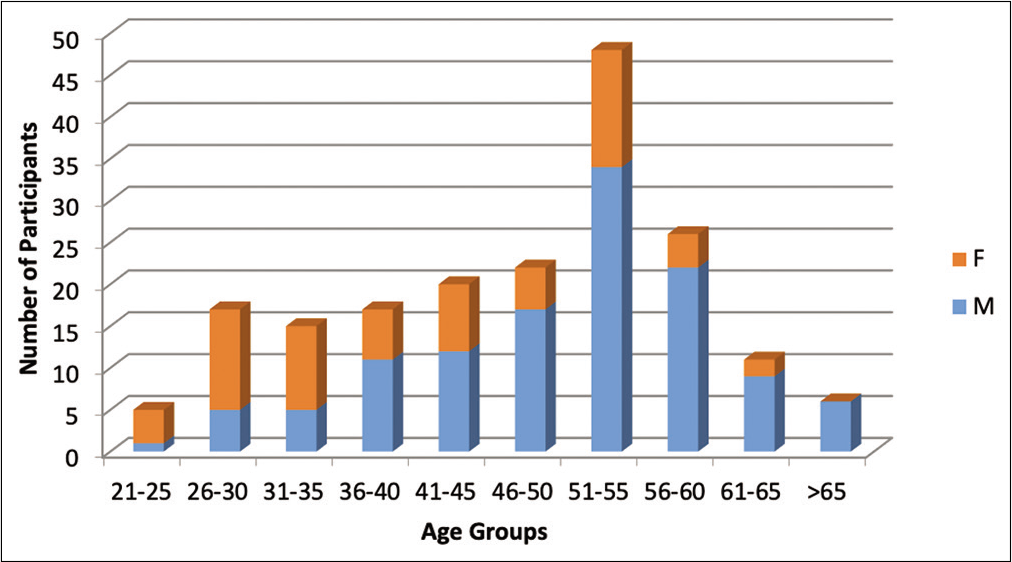
- Demographics of the study population.
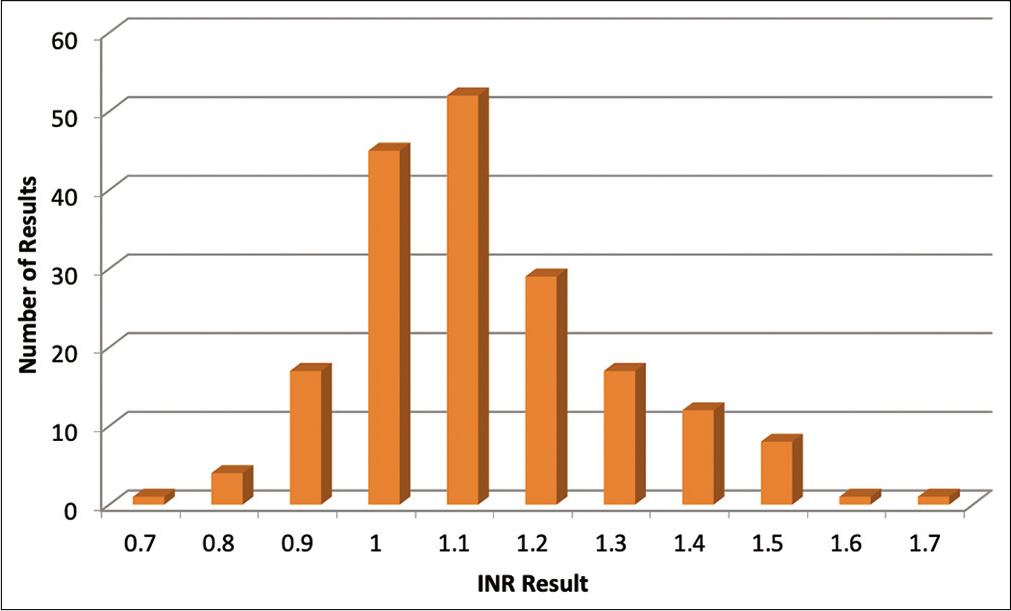
- Graphical representation of the incidence of international normalized ratio values in liver disease patients who received invasive dental care.
| Mean | Median | Mode | Range | Standard deviation | Control | Z-score | P-value | Confidence interval (μ) |
|---|---|---|---|---|---|---|---|---|
| 1.126 | 1.1 | 1.1 | 0.7–1.7 | 0.17 | 1.2 | 0.43529412 | 0.332181 | 1.126±0.02443 |
Confidence interval for P value based on confidence level of 95% was also calculated and result indicates that the mean value falls between 1.10157 and 1.15043.
DISCUSSION
When providing treatment for patients with liver diseases, it is essential that clinicians perform thorough history and physical examinations and should not depend entirely on previous or current laboratory values to assess bleeding risk.[3,11]
If concerned for intra- or post-operative bleeding in patients with suspected liver disease, an examination should also include history of disease, risk factors for chronic liver diseases, and history of previous bleeding during or following a surgical procedure.[3] Clinical presentation or history of ascites, jaundice, or encephalopathy should be considered indicators of possible increased bleeding risk.[6] In patients with chronic liver diseases, reduced production of procoagulant factors is often balanced by decreased production of anticoagulant factors synthesized by the liver including antithrombin, protein C, and protein S.[8,9,11,12]
Procoagulant proteins are normally present in excess requiring severe reduction before resulting in hemorrhage and in contrast, a more modest reduction of anticoagulant proteins can severely impair the antithrombotic potential of plasma9. Many patients with liver diseases appear to achieve balance between reduced pro- and anti-coagulant levels and neither have hemorrhage nor thrombosis.[11,13-15]
In a cornerstone study in 2006 by Ward and Weidmen, INR values were not found to be statistically significant in predicting postoperative bleeding.[13] This can be attributed to the fact that widely used coagulation tests may not reflect the activity of procoagulant and anticoagulant factors in vivo.[8]
INR was designed to monitor patients on VKA and does not pick up the anticoagulant changes in liver disease patients.[11] Thus, it is important that INR test results for patients taking warfarin should not be misinterpreted as having the same meaning as the INR of patients with liver diseases.[3,16] The coagulation defects mediated by VKA anticoagulants and chronic liver diseases are different.[8,17,18] In addition, thrombin in patients with stable liver disease is similar to healthy individuals.[19] To downregulate thrombin generation, the major anticoagulant mechanism operating in vivo including protein C which mediates its anticoagulant effects by forming a thrombin-thrombomodulin complex needs to be completely activated; however, the time interval from the onset of coagulation activation to fibrin formation is so rapid that is not possible for this process to occur effectively.[9] In normal homeostasis, antithrombin regulates the primary inhibitor activity and is amplified by glycosaminoglycans located on endothelial cells, but the plasma utilized in coagulation tests does not contain thrombomodulin or glycosaminoglycans.[8] Although INR provides information whether a patient is deficient in procoagulant proteins, it does not imply if that deficiency is compensated within the system.[19] Despite the known diminishing role of INR for many years, there are still health-care providers who depend heavily on INR to make decision whether to provide dental treatment for patients with liver diseases who are not on VKA medications. In our study, there was no significance difference between the INR value of patients with liver diseases, excessive alcohol consumption, or abnormal liver function tests and with normal INR range. Numerous studies demonstrate that the use of PT-INR when used in patients with liver diseases has substantial interlaboratory variation.[20-24] The risk of bleeding is difficult to predict with patients with liver failure, but the frequency of delayed bleeding is generally low.[25] It has been suggested that patients with active liver disorders, such as cirrhosis, who require oral surgery procedures should be managed in hospital clinics, where access to hematological assessment and appropriate surgical and medical care are readily available.[26] In any clinical setting, however, local hemostatic measures are effective method for achieving hemostasis in oral surgical procedures even in candidates for liver transplant.[27,28]
CONCLUSION
The results of this study show that INR values of patients with liver diseases who are not taking VKA, are not statistically different from normal range, and therefore, INR is not a dependable marker to predict the bleeding risk intra and postoperatively in this group of patients . Hence, in the absence of clinical signs of liver diseases or liver failure, the use of INR as a prerequisite for surgical dental procedures in the patients with chronic liver diseases or abnormal liver function tests, who are not taking VKA anticoagulants, is both arbitrary and should not be required. In fact, it might provide a false sense of security to the oral health-care providers and may be considered an overuse of resources. This is also an affirmation that clinicians should spend more time on history and physical evaluation than on laboratory studies for these patients and not to make treatment decision entirely based on INR, the results of which may not be appropriately interpreted.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Sleisenger and Fordtran's Gastrointestinal and Liver Disease (7th ed). Philadelphia, PA: Saunders; 2002. p. :1216-20.
- [Google Scholar]
- Hemostatic disorders in liver disease In: Schiff ER, Sorrell MF, Maddrey WC, eds. Diseases of the Liver. Philadelphia, PA: Lippincott Williams and Wilkins; 2003. p. :625-36.
- [Google Scholar]
- Dental postoperative bleeding complications in patients with suspected and documented liver disease. Oral Dis. 2012;18:661-6.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of chronic hepatitis B virus (HBV) infection in U.S. households: National health and nutrition examination survey (NHANES), 1988-2002 Hepatology. . 2016;63:388-97.
- [CrossRef] [PubMed] [Google Scholar]
- Surveillance for viral hepatitis In: Division of Viral Hepatitis and National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention. United States: Centers for Disease Control and Prevention; 2016.
- [Google Scholar]
- Lockhart utility of an international normalized ratio testing device in a hospital-based dental practice. JADA. 2008;139:697-703.
- [CrossRef] [PubMed] [Google Scholar]
- Risk assessment of long term post-operative bleeding following tooth extraction(s) in the pre-transplant liver failure patient. AAOMS. 2005;63:33-4.
- [CrossRef] [Google Scholar]
- The international normalized ratio to prioritize patients for liver transplantation: Problems and possible solutions. J Thromb Haemost. 2008;6:243-8.
- [CrossRef] [PubMed] [Google Scholar]
- The prothrombin time test as a measure of bleeding risk and prognosis in liver disease. Aliment Pharmacol Ther. 2007;26:141-8.
- [CrossRef] [PubMed] [Google Scholar]
- Reference Ranges from Quest Diagnostic, Cleveland Clinic, University of Wisconsin. Available from: https://www.testdirectory.questdiagnostics.com/test/test-detail/4914/?cc=master https://www.my.clevelandclinic.org/health/diagnostics/17691-prothrombin-time-pt-test/results-and-follow-up https://www.ucsfhealth.org/medical-tests/003652 https://www.uwhealth.org/lab-test-directory/coagulation/name-67687-en.html https://www.abem.org/public/docs/default-source/default-document-library/laboratory-normal-values.pdf?sfvrsn=2
- [Google Scholar]
- Thrombin generation in patients with cirrhosis: The role of platelets. Hepatology. 2006;44:440-5.
- [CrossRef] [PubMed] [Google Scholar]
- Evidence of normal thrombin generation in cirrhosis despite abnormal conventional coagulation tests. Hepatology. 2005;41:553-8.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term postoperative bleeding after dentoalveolar surgery in the pre-transplant liver failure patient. J Oral Maxillofac Surg. 2006;64:1469-74.
- [CrossRef] [PubMed] [Google Scholar]
- Liver biopsy bleeding time: An unpredictable event. J Gastroenterol Hepatol. 1994;9:269-71.
- [CrossRef] [PubMed] [Google Scholar]
- Paucity of studies to support that abnormal coagulation test results predict bleeding in the setting of invasive procedures: An evidence-based review. Transfusion. 2005;45:1413-25.
- [CrossRef] [PubMed] [Google Scholar]
- Interpretation of the international normalized ratio in patients with liver disease. Lancet. 2002;359:47-8.
- [CrossRef] [Google Scholar]
- Haemostatic abnormalities in patients with liver disease. J Hepatol. 2002;37:280-7.
- [CrossRef] [Google Scholar]
- The international normalized ratio (INR) in the MELD score: Problems and solutions. Am J Transplant. 2010;10:1349-53.
- [CrossRef] [PubMed] [Google Scholar]
- Normal to increased thrombin generation in patients undergoing liver transplantation despite prolonged conventional coagulation tests. J Hepatol. 2010;52:355-61.
- [CrossRef] [PubMed] [Google Scholar]
- Specific laboratory methodologies achieve higher model for endstage liver disease (MELD) scores for patients listed for liver transplantation. Liver Transplantation. 2004;10:995-1000.
- [CrossRef] [PubMed] [Google Scholar]
- Inter-laboratory variation in INR leads to clinically relevant changes in MELD score: Survey of US clinical laboratories. J Transplantation. 2006;82(Suppl 2):333.
- [CrossRef] [Google Scholar]
- Prothrombin time in liver failure: Time, ratio, activity percentage, or international normalized ratio. Hepatology. 1996;24:1392-4.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment of the validity of the INR system for patients with liver impairment. Thromb Haemost. 1994;71:727-30.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative studies of rabbit and human recombinant tissue factor reagents. Thromb Res. 1999;94:255-61.
- [CrossRef] [Google Scholar]
- Oral surgery in liver transplant candidates: A retrospective study on delayed bleeding and other complications. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;121:490-5.
- [CrossRef] [PubMed] [Google Scholar]
- Persistent haemorrhage following dental extractions in patients with liver disease: Two cautionary tales. Br Dent J. 1996;180:141-4.
- [CrossRef] [PubMed] [Google Scholar]
- Postoperative bleeding after dental extraction in liver pretransplant patients. J Oral Maxillofac Surg. 2012;70:e177-84.
- [CrossRef] [PubMed] [Google Scholar]
- Bleeding during and after dental extractions in patients with liver cirrhosis. Int J Oral Maxillofac Surg. 2018;47:1543-9.
- [CrossRef] [PubMed] [Google Scholar]






