Antibacterial efficacy of spice extracts against Streptococcus mutans and Lactobacillus acidophilus – An in-vitro study

*Corresponding author: Pooja Latti, Department of Public Health Dentistry, Annoor Dental College and Hospital, Muvattupuzha, Kerala, India. drpooja_l@yahoo.co.in
-
Received: ,
Accepted: ,
How to cite this article: Latti P, Subramaniam R, Prashant GM. Antibacterial efficacy of spice extracts against Streptococcus mutans and Lactobacillus acidophilus – An in vitro study. J Global Oral Health 2021;4:73-7.
Abstract
Objectives:
To evaluate the antibacterial activity of black pepper, Indian bay leaf, cinnamon, and cumin against Streptococcus mutans and Lactobacillus acidophilus in-vitro and to determine their minimum inhibitory concentration (MIC).
Materials and Methods:
The spices (cinnamon, cumin, Indian bay leaf, and black pepper) were obtained from local market, were dried and powdered. Solvent extracts were prepared with methanol by maceration, followed by filtration and evaporation. The antimicrobial activity was assessed using cup plate diffusion method, followed by determination of MIC of the extracts. Statistical analysis was performed using one-way analysis of variance and Tukey’s post hoc test was used for pairwise comparison. P < 0.05 was considered statistically significant.
Results:
All the four extracts showed significant antimicrobial activity. Cinnamon demonstrated maximum activity against S. mutans (zone of inhibition of 18.1 mm ± 0.30) and L. acidophilus (zone of inhibition of 17.9 mm ± 0.44) with the least MIC against the organisms (<0.05 mg/ml).
Conclusion:
All the spice extracts tested demonstrated significant antibacterial activity against S. mutans and L. acidophilus. On comparison of the antibacterial activities of all the four extracts, cinnamon extract emerged as the potent agent.
Keywords
Cinnamon bark (Cinnamomum verum)
Cumin (Cuminum cyminum)
black pepper (Piper nigrum)
Indian bay leaf (Cinnamomum tamala)
Streptococcus mutans
Lactobacillus acidophilus
Antibacterial
INTRODUCTION
Dental caries has always been associated with the dental profession, an association that is punctuated with agony and ecstasy. The agonizing fact is that despite several efforts toward total eradication, this disease is still prevalent.[1]
The oral cavity consists of wide variety of bacteria, but only a few specific species of bacteria cause dental caries: Streptococcus mutans and Lactobacilli are among the most important bacteria.[2]
S.mutans are non-motile Gram positive, anaerobic cocci which metabolizes carbohydrates and are therefore the primary etiological agents of dental caries.[3] The cariogenicity is due to various factors including dextran, production of acid in the plaque, and glucosyltransferase enzyme activity. Water insoluble glucans are significant constituents of dental plaque biofilms that facilitate adherence and accumulation of S.mutans and other oral bacteria. The biofilm formation is influenced by the amount of glucosyltransferase produced by S.mutans. the most common pathogen isolated from human dental plaque and their prevalence has been reported in several epidemiological studies.[2,4]
Lactobacilli may be found in the mouth before the teeth erupt; and they are known to preferentially colonize the dorsum of the tongue and are also carried into saliva by the sloughing of the tongue’s epithelium. Their numbers in saliva reflects the consumption of simple carbohydrates by the host. They are highly acidogenic from carbohydrates and are acid tolerant, and they are often cultured from established carious lesions.[3]
Since the discovery of S.mutans and Lactobacilli as major etiological agents for dental caries, these bacteria received attention, as a target for prevention of the dental caries through the use of vaccines and antimicrobial agents. Application of antibiotics for prevention and treatment of dental caries would be harmful for the patients and the development of multidrug-resistant strains of bacteria is also possible.[4]
A possible way to overcome the problem of microbial resistance is the development of new antibacterial agents. Thus, the recent global attention has shifted toward finding of new chemicals, especially spices, for the development of new drugs. Studies indicate that natural products are important sources for new drugs. Plant-based medicines, now serve as the basis of novel drug discovery.[5]
Thus, the objectives of the study were to investigate the in vitro antibacterial activity of alcoholic extracts of black pepper, Indian bay leaf, cinnamon, and cumin against S.mutans and Lactobacillus acidophilus and to determine the minimum inhibitory concentration (MIC) of the spice extracts against the test organisms.
MATERIALS AND METHODS
Plant materials
Cinnamon bark (Cinnamomum verum), cumin (Cuminum cyminum), dried black pepper fruits (Piper nigrum), and dried Indian bay leaves (Cinnamomum tamala) were obtained from the local market of Davanagere city, Karnataka, India. All the specimens were identified by a botanist and authenticated by a pharmacognosist at the Department of Pharmacognosy, Bapuji College of Pharmacy, Davanagere.
The spices were cleaned and washed with sterile distilled water. The spices were further air-dried on filter paper at room temperature and then powdered using a mechanical grinder and then were passed through a sieve so that uniform powder size was obtained.
Preparation of the extract
Solvent extracts were prepared by maceration technique[6] with methanol. Ten grams of ground spice were added to 100 ml methanol and agitated at room temperature for 8 h in a wrist-action shaker. Thereafter, the mixture was allowed to stand for 12 h and the mixture thus obtained was concentrated by complete evaporation of solvent at room temperature to yield the pure extract [Figure 1]. Pure solutions of crude extracts with 100% concentration were prepared by mixing well the appropriate amount of dried extracts with an inert solvent di-methyl sulfoxide (negative control). For determination of MICs, appropriate dilutions of the extracts were prepared by mixing well the appropriate amount of dried extracts with the inert solvent.

- Solvent extracts of the spices.
Microorganism
The microorganisms selected for the study were S.mutans (ATCC 25175) and L.acidophilus (ATCC 4356). The microbial strains were obtained from American Type Culture Collection, United States of America. The organisms were sub-cultured on brain heart infusion (BHI) agar at 37°C for 48 h and the stock culture was maintained at 4°C.
Screening for antibacterial activity
Cup plate diffusion method[7]
The antibacterial activity of the spice extracts was assessed by the cup plate diffusion method. Petri dishes containing 18 ml of BHI Agar for S.mutans and L.acidophilus were poured. One hundred microliters of inoculum of each of the test organisms were spread onto the specific media plates to achieve a confluent growth. The agar plates were allowed to dry. Wells of 8 mm diameter were made with a sterile device. A 100 μl volume of each extract was propelled directly into the wells (in triplicates) of the inoculated specific media agar plates. The plates were kept idle for 10 min for diffusion of the extract to take place and the plates were incubated at 37°C for 24 h. The experiment was performed in triplicate to ensure consistency of all findings [Figure 2].
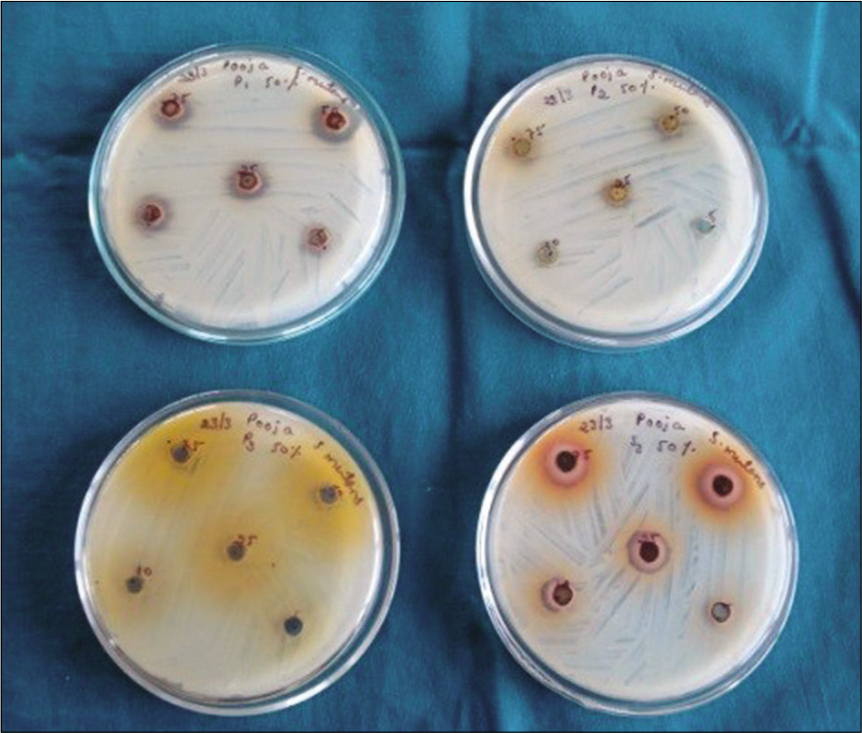
- Determination of zone of inhibition by disk diffusion.
After incubation for 24 h at 37°C, the plates were observed. If antibacterial activity was present on the plates, it was indicated by clear zones of growth inhibition surrounding the well containing the extract. Vernier calipers were used to measure zone of inhibition and expressed in millimeters.
Determination of the MIC
The MIC was reported as the lowest concentration of the extracts capable of inhibiting the growth of the bacterium tested.[8] In the present study, MIC was determined using “Serial tube dilution technique.”[9] Ten test tubes were taken, and labeled from 1 to 10. One millimeter of BHI broth was poured into each of the test tubes. The test tubes were plugged with cotton and sterilized using an autoclave. After cooling, 1 ml of the sample was added to the 1st test tube and mixed well to obtain a concentration of 50%. The tubes were serially diluted until a concentration of 0.1% was obtained in the 10th test tube. Ten microliters of properly diluted test organism inoculum were added to each of ten test tubes and mixed well. All the test tubes were incubated at 37°C for 24 h. Following incubation, the MIC was calculated as the lowest concentration of the extracts inhibiting the visible growth of the bacterial strain using a reflective viewer [Figure 3].
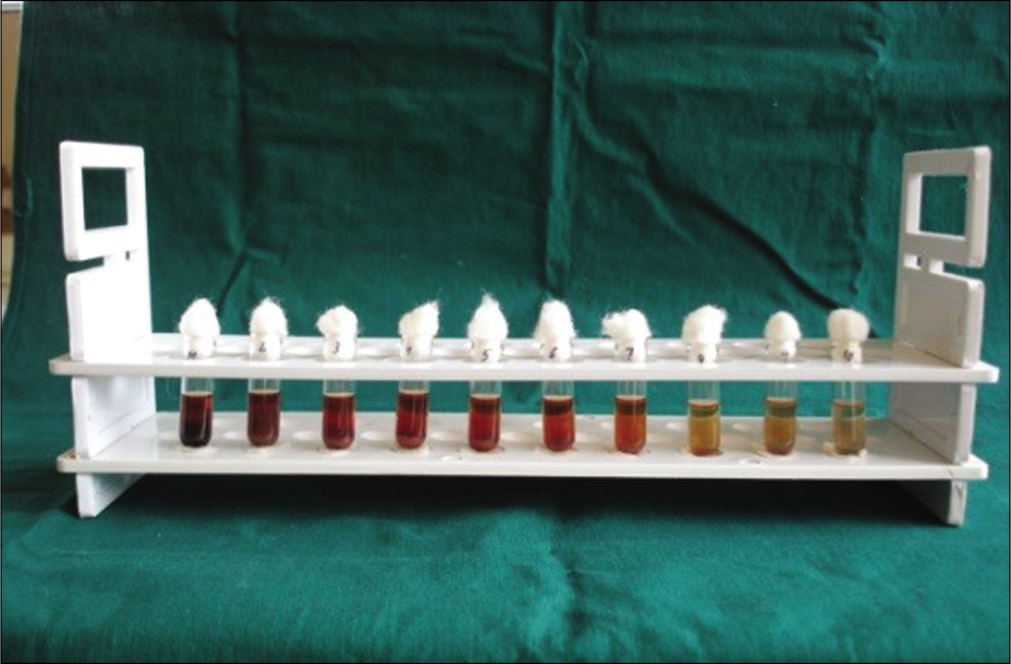
- Determination of minimum inhibitory concentration through serial tube dilution technique.
Statistical analysis
Data collected by experiments were tabulated and analyzed using the Statistical Package for the Social Sciences (SPSS version 17.0). One-way analysis of variance test was used for multiple group comparisons, followed by Tukey’s post hoc for pair-wise comparisons. P < 0.05 was considered statistically significant.
RESULTS
The results of antibacterial activity of the methanolic extracts of black pepper, Indian bay leaf, cinnamon, and cumin by cup plate diffusion method revealed that all the four extracts showed significant antibacterial activity against S.mutans and L.acidophilus. In the present study, significant differences in the mean zone of inhibition observed among all the four spice extracts. The antibacterial activity of the four extracts against S.mutans and L.acidophilus is summarized in [Tables 1 and 2]. The results of the MIC are summarized in [Table 3].
| Sl. no. | Spice extracts | Mean zone of inhibition (mm) | Analysis of variance | Tukey’s post hoc |
|---|---|---|---|---|
| 1. | Cinnamon | 18.1±0.30 | F=296.14 | Cinnamon > Indian bay leaf > |
| 2. | Black pepper | 14.9±0.41 | P<0.001 (Significant) | Black pepper > Cumin > DMSO |
| 3. | Cumin | 13.8±0.36 | ||
| 4. | Indian bay leaf | 16.9±0.41 | ||
| 5. | Dimethyl sulfoxide (DMSO) | No inhibition |
| Sl. no. | Spice extracts | Mean zone of inhibition (mm) | Analysis of variance | Tukey’s post hoc |
|---|---|---|---|---|
| 1. | Cinnamon | 17.9±0.44 | F=357.598 | Cinnamon > Black pepper > Indian bay leaf> Cumin > DMSO |
| 2. | Black pepper | 15.9±0.48 | P<0.001 | |
| 3. | Cumin | 11.9±0.47 | (Significant) | |
| 4. | Indian bay leaf | 15.8±0.43 | ||
| 5. | Dimethyl sulfoxide (DMSO) | No inhibition |
| Sl. no. | Name of extracts | MIC against Streptococcus mutans(mg/ml) | MIC against Lactobacillus acidophilus(mg/ml) |
|---|---|---|---|
| 1. | Cinnamon | <0.05 | <0.05 |
| 2. | Black pepper | 1.6 | 0.2 |
| 3. | Cumin | 3.12 | 6.25 |
| 4. | Indian bay leaf | 0.4 | 0.2 |
DISCUSSION
Conventionally, herbs and spices are part of routine Indian food preparations as they make food appealing by providing better appearance, smell, and taste. As mentioned in “Atharva veda,” these herbs and spices have healing, soothing, and rejuvenating properties.[10] Some of the common herbs and spices used are chili, clove, coriander, mint, garlic, ginger, turmeric, tulsi, cinnamon, tamarind, asafoetida, black pepper, Indian bay leaf, cumin, etc.
“Ayurveda” the indigenous system of Indian medicine, uses a large number of spices in combinations as preventive and curative medicines.[10] There are a number of reports available in literature stating the sensitivity of various bacteria toward these herbs and spices.[11,12]
The present study demonstrates the antimicrobial activity of solvent extracts of cinnamon, black pepper, cumin, and Indian bay leaf against S. mutans and L. acidophilus.
Organic solvent (methanol) rather than distilled water was used in the preparation of plant extracts. The polarity of antibacterial compounds make them more readily extracted by organic solvents, and using organic solvents do not negatively affect their bioactivity against bacterial species.[5]
Cinnamon has been used as a spice in several cultures for centuries. It was, traditionally, used mainly as a stomachic and carminative for gastrointestinal complaints and is still used for these conditions today. The bark of Cinnamomum zeylanicum and Cinnamomum cassia is used as spice (cinnamon bark). These two species are the only approved medicinal herbs of the genus Cinnamomum.[13] The results of the present study revealed that solvent extract of cinnamon possesses appreciable potentiality of inhibiting the growth of S.mutans. C. cassia as the bark contains cinnamaldehyde, cinnamic acid, cinnamyl alcohol, and coumarin.[13] Cinnamaldehyde is an aromatic aldehyde that inhibits amino acid decarboxylase activity. Cinnamon bark is rich in cinnamaldehyde (50.5%), which is highly electronegative. Such electronegative compounds interfere in biological processes involving electron transport and react with nitrogen-containing compounds, for example, proteins and nucleic acids and inhibit the growth of microorganisms.[14] Thus, the antimicrobial activity of cinnamon may be attributed to the presence of these compounds. The results of the present study are in concordance with the research carried out on the same species by Chaudhry et al., in 2006,[15] and Rezwani et al., in 2017,[16] and on other species of same genera by Aneja et al., in 2009.[17] A study conducted by Chaudhari in 2012 revealed high activity of cinnamon oil on S.mutans.[18]
In the present study, cinnamon demonstrated antibacterial activity against L.acidophilus. Earlier reports by Aneja et al., Haryana, India, 2009, indicated no activity of cinnamon extract on L.acidophilus.[17]
The results from the present study demonstrated that black pepper potentially inhibited the growth of S.mutans. The observed antibacterial activity may be attributed to the volatile oils of pepper which has been shown to have antimicrobial activity.[19] The results of our study are in agreement with the observations of the previous researches conducted by Chaudhry et al.[11] and Sweta et al.[20]
In the present study, cumin demonstrated antibacterial activity against S.mutans. The antibacterial activity of C.cyminum essential oil is perhaps attributable to the high level of cumin aldehyde (16.1%), a compound with known antimicrobial properties, and to α-pinene, the other main component (11.4%) of C.cyminum essential oil, which inhibited the growth of bacteria.[21] Similar results have been obtained in studies conducted by Ghazi et al.[22] and Al Shawi et al.[23] The results of the present study are in contrast with the study conducted by Chaudhry et al., Karachi, Pakistan, 2008.[24] This contrasting result might be due to the differences in the extraction procedure.
In this study, Indian bay leaf exhibited antibacterial activity against both S.mutans and L.acidophilus. The antibacterial role may be due to the active constituents mainly eugenol and tannins.[25,26] A study conducted on aqueous extract of bay leaf demonstrated an antibacterial activity against S.mutans.[26]
CONCLUSION
All the spice extracts tested demonstrated significant antibacterial activity. On comparison of the antibacterial activities of all the four extracts, cinnamon extract emerged as the potent agent. Hence, the need of the hour is to perform more and more screening of the natural products or plant parts as such screening experiments form a primary platform for further pharmacological and phytochemical studies that may open the possibilities of finding new clinically effective antibacterial compounds against the dental caries pathogens.
Acknowledgment
We would like to acknowledge Dr. Kishore Bhat, Professor, Department of Microbiology, Maratha Mandal Medical College, Belagavi and Dr. K. S. Muralikrishna, Principal, MLR Institute of Pharmacy, Telangana, for their help in this research.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Dental caries-a complete changeover (Part I) J Conserv Dent. 2009;12:46-54.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of oil-pulling on dental caries causing bacteria. Afr J Microbiol Res. 2008;2:63-6.
- [Google Scholar]
- The microbiology of primary dental caries in humans. J Dent Educ. 2001;65:1028-38.
- [CrossRef] [PubMed] [Google Scholar]
- Inhibitory activity of garlic (Allium sativum) extract on multidrug-resistant Streptococcus mutans. J Indian Soc Pedod Prev Dent. 2007;25:164-8.
- [CrossRef] [PubMed] [Google Scholar]
- Laboratory evaluation of crude extracts of Cinnamomum tamala for potential antibacterial activity. Electron J Biol. 2009;5:75-9.
- [Google Scholar]
- Techniques for extraction and isolation of natural products: A comprehensive review. Chin Med. 2018;13:20.
- [CrossRef] [PubMed] [Google Scholar]
- Analytical microbiology. II. The diffusion methods. Appl Microbiol. 1957;5:25-33.
- [CrossRef] [PubMed] [Google Scholar]
- Antimicrobial activity of some spices against selected microbes. Int J Pharm Pharm Sci. 2010;2:187-96.
- [Google Scholar]
- Determination of Minimum Inhibitory Concentration (MIC) of the Isolated Pure Compounds. Available from: http://www.jesbd.info/mic.pdf [Last accessed on 2020 Aug 15]
- [Google Scholar]
- Antibacterial effect of herbs and spices extract on Escherichia coli. Electron J Biol. 2009;5:40-4.
- [Google Scholar]
- Bactericidal activity of black pepper, bay leaf, aniseed and coriander against oral isolates. Pak J Pharm Sci. 2006;19:214-8.
- [Google Scholar]
- Antimicrobial, antifungal and insecticidal investigations on essential oils: An overview. Nat Prod Radiance. 2005;4:179-92.
- [Google Scholar]
- Cinnamon (Cinnamomum spp.) Available from: http://www.naturalstandard.com [Last accessed on 2020 Aug 16]
- [Google Scholar]
- Comparative analysis of the antimicrobial activity of cinnamon oil and cinnamon extract on some food-borne microbes. Afr J Microbiol Res. 2008;2:247-51.
- [Google Scholar]
- Anti-microbial activity of Cinnamomum cassia against diverse microbial flora with its nutritional and medicinal impacts. Pak J Bot. 2006;38:169-74.
- [Google Scholar]
- The synergestic effect of honey and cinnamon against Streptococcus mutans bacteria. Asian Pac J Trop Med. 2017;7:314-20.
- [CrossRef] [Google Scholar]
- Antimicrobial activity of Dalchini (Cinnamomum zeylanicum bark) extracts on some dental caries pathogens. J Pharm Res. 2009;2:1387-90.
- [Google Scholar]
- Antimicrobial activity of commercially available essential oils against Streptococcus mutans. J Contemp Dent Pract. 2012;13:71-4.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative chemical composition and antimicrobial activity of berry and leaf essential oils of Piper nigrum L. Int J Biol Med Res. 2010;1:215-8.
- [Google Scholar]
- In vitro antibacterial activity of clove and pepper on Streptococcus mutans. Asian J Pharm Res. 2015;8:269-70.
- [Google Scholar]
- Antibacterial activity of Cuminum cyminum L and Carum carvi L essential oils. J Agric Food Chem. 2005;53:57-61.
- [CrossRef] [PubMed] [Google Scholar]
- Investigation of antibacterial effect of Cumunum cyminum and Carum carvi against Streptococcus mutans and Streptococcus pyogenes. Novelty Biomed. 2017;7:1.
- [Google Scholar]
- Study of cumin antibacterial and antioxidant activity of alcoholic and aqueous extracts. Pak J Biotechnol. 2017;14:227-31.
- [Google Scholar]
- In vitro antibacterial activities of kalonji, cumin and poppy seed. Pak J Bot. 2008;40:461-7.
- [Google Scholar]
- In vitro-antibacterial activity and phytochemical profiles of Cinnamomum tamala (Tejpat) leaf extracts and oil. Rev Infect. 2010;1:134-9.
- [Google Scholar]
- Effectiveness of bay leaves aqueous extract on Streptococcus mutans in comparision to chlorhexidine gluconate. IOSR J Pharm Biol Sci. 2017;12:12-6.
- [Google Scholar]






