Translate this page into:
Tumor-associated tissue eosinophilia in oral squamous cell carcinoma – A predictable biological behavior

-
Received: ,
Accepted: ,
How to cite this article: Shrestha P, Narayan K, Kumar VV, Hemadala GC, Murgod S. Tumor-associated tissue eosinophilia in oral squamous cell carcinoma – A predictable biological behavior. J Global Oral Health 2020;3(1):3-8.
Abstract
Objectives:
Squamous cell carcinoma of the oral cavity is the most common aggressive epithelial malignant neoplasm. Its biological behavior is influenced by the host immune cells, such as multifaceted eosinophils, associated with wound healing and tissue damage processes. Their presence within a variety of human cancers raises queries about their role. The infiltrations of tumor stroma by eosinophils are believed to play a significant role in progression of the carcinoma and could be either a potential diagnostic tool for stromal invasion or as a prognostic indicator. Its role in cancer still remains unclear since in the literature, there are very few studies showing improved prognosis and few contradictory studies showing poor prognosis. This study was conducted with an aim to compare the tumor-associated tissue eosinophilia in oral squamous cell carcinoma (OSCC) and normal tissue and to correlate the expression in different grades of carcinoma using a special stain that targets eosinophils exclusively and vividly.
Materials and Methods:
The study includes 30 samples, six normal, and 24 histopathologically diagnosed with OSCC. 5 μ thick sections were made and stained using special stain and examined under high power (×40), ten consecutive microscopic fields were studied. The average numbers of eosinophils were statistically analyzed.
Results:
Eosinophil count for carcinoma was higher compared to normal mucosa, but the comparison in different grades of cancer did not show much difference.
Conclusion:
Since eosinophil count was higher in carcinoma, eosinophil infiltration in dysplastic cases should prompt thorough evaluation for invasiveness.
Keywords
Eosinophils
Tumor-associated tumor eosinophilia
Oral squamous cell carcinoma
Carbol chromotrope
Special stains
INTRODUCTION
Cancers of the oral and paraoral region remain a severe and rapidly progressing life-threatening disease in many parts of the world.[1] Oral squamous cell carcinoma (OSCC) is a very aggressive neoplasm[2] constituting approximately 5–8% of all head-and-neck cancers globally.[1] About 30% of all new cases and 22.9% of deaths arising annually, oral cancer remains the sixth most common cancer in the world, with male predominance and the third most among females in the Indian subcontinent with high mortality and morbidity rates between the 6th and 8th decades of life.
Cancer-associated stroma is a complex extracellular matrix environment, where a variety of interactions between tumor and stromal cells takes place. Inflammatory cells such as lymphocytes, plasma cells, neutrophils, macrophages, mast cells, and eosinophils arrive at the tumor site due to the host response mechanism that varies from individual to individual.[3] Increased tissue eosinophil levels have been reported in various malignancies, including OSCC.[4] Wharton Jones, in 1846, first observed a rare granulocyte with coarse granules termed as “eosinophils” by Paul Ehrlich later in 1880.[5] These multifunctional, multifaceted leukocytes play a key role in health and disease, are also involved in the pathogenesis, inflammatory processes, infectious diseases, tissue injury, tumor immunity, and allergic reactions.[6]
Infiltration of eosinophils in the tumor environment plays an important role in biological behavior of cancer such as carcinoma stromal interactions. Tumor-associated tissue eosinophilia (TATE) is characterized by the presence of eosinophils as a component of peritumoral and intratumoral inflammatory infiltrate.[7] Over the years, numerous studies have been directed toward the identification of role of these cells either in the pathogenesis or in the tumor development process. Few available studies suggest favorable prognosis in tumors with tissue eosinophilia, while others suggest eosinophils play a role in promoting epithelial tumor growth.[8]
Routinely, TATE in OSCC’s is counted after staining with hematoxylin and eosin. Intact eosinophils can usually be detected in tissue sections of tumors. In some cases, tissue eosinophils often assume an amoeboid or “medusa” cell configuration, especially in fibrous tissue, making their recognition very difficult. Hence, in this study, carbol chromotrope was used, with a benefit that it selectively and vividly stains eosinophils.[6] The aim of this study is to compare the tissue eosinophils in OSCC and normal tissue and to correlate the expression of TATE in different grades of OSCC.
MATERIALS AND METHODS
The study was carried out with sample size of 30 cases, of which 24 were OSCC, and six were normal tissue. Tissue blocks were taken from archives of the department of oral pathology and microbiology of a teaching dental institution. The samples of eight well-differentiated squamous cell carcinoma (WDSCC), eight moderately differentiated squamous cell carcinoma (MDSCC), and eight poorly differentiated squamous cell carcinoma (PDSCC) were taken after histopathological diagnosis.
5 μ thick sections were made and stained using special stain – carbol chromotrope. The section was dewaxed and brought to water. Then, it was stained with Mayers hematoxylin for 8 min and bluing was done. It was then placed in carbol chromotrope staining solution for 30 min at room temperature and then washed with tap water. Then, it was dehydrated in alcohol, cleared in xylene, and mounted with DPX.
Eosinophilic granules stained bright red with carbol chromotrope. These cells were then observed under compound microscope.
Counting of tumor-associated tissue eosinophils[9]
There are basically two methods described for eosinophil counting. In classical counting, eosinophils are counted per high-power field (hpf) in sections of tumor or tumor edge or surrounding stroma randomly. In density method, the highest eosinophil density per surface area is counted using grid of definite dimensions.
Eosinophils were counted by classical method[9] which was done by randomly selecting ten high-power fields in each slide which showed high density of these cells. Each field was screened under ×40 objective lens using “zigzag” method for the evaluation of TATE. Eosinophils per 10 hpf were noted for TATE in normal mucosa [Figure 1], WDSCC [Figure 2], MDSCC [Figure 3] and PDSCC [Figure 4]. A total numbers of cells were counted and divided by total number of fields to obtain an average number of cells. Data were collected, tabulated, and subjected to statistical analysis using Mann–Whitney test and Kruskal–Wallis non-parametric test to compared eosinophils in grades of OSCC.
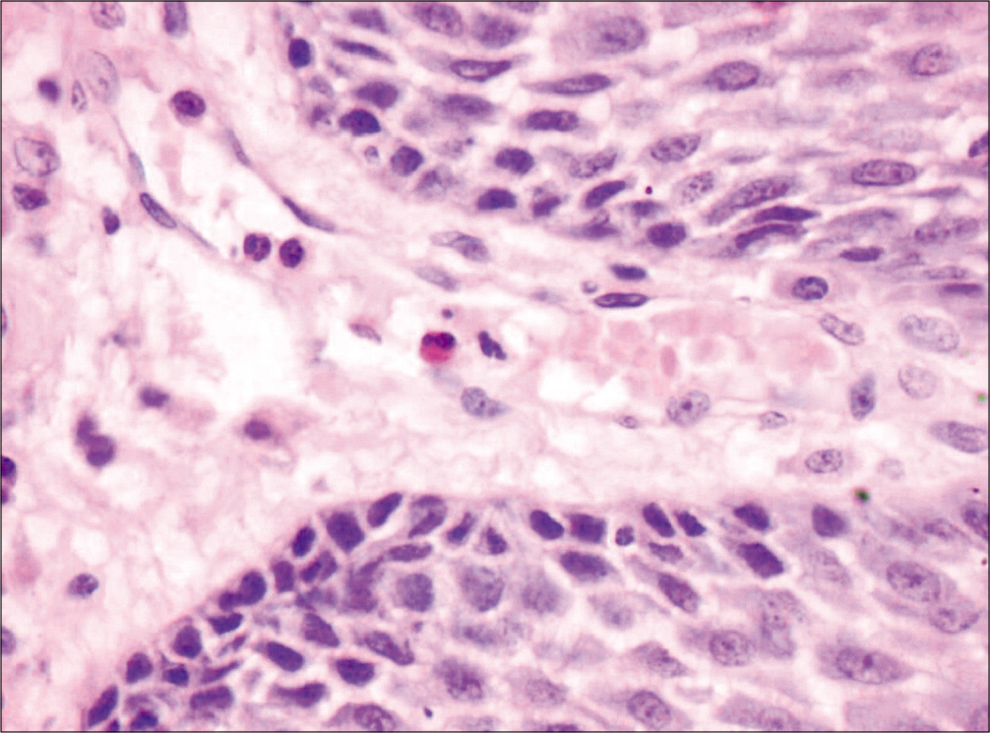
- Normal mucosa (×40), carbol chromotrope stain.
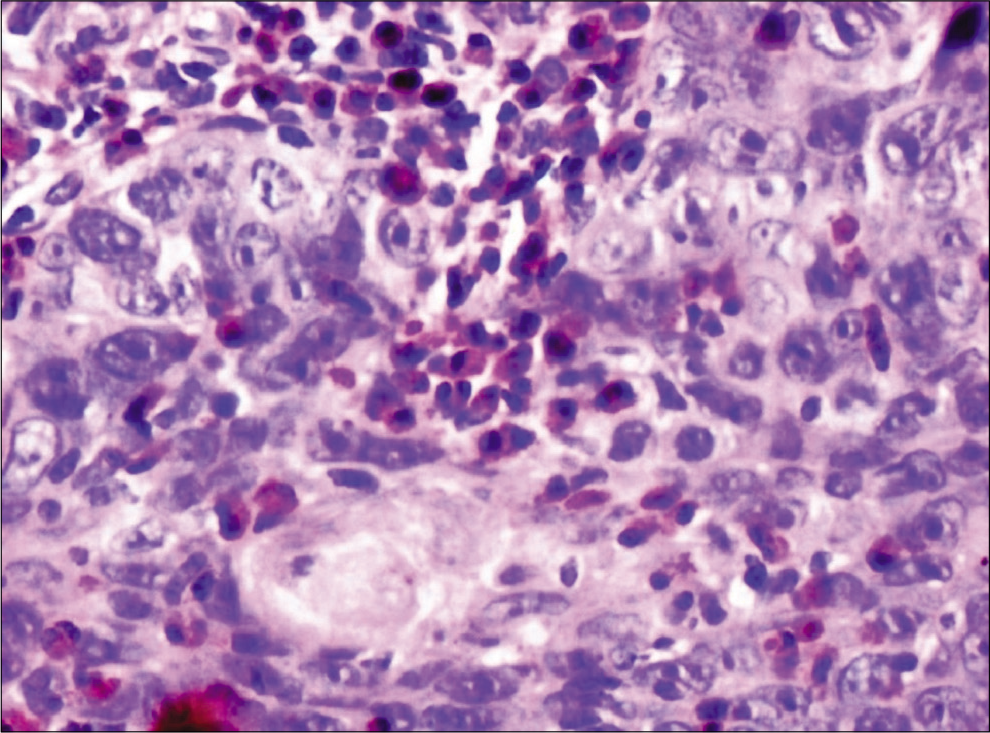
- Tumor-associated tissue eosinophilia in well-differentiated squamous cell carcinoma (×40), carbol chromotrope stain.
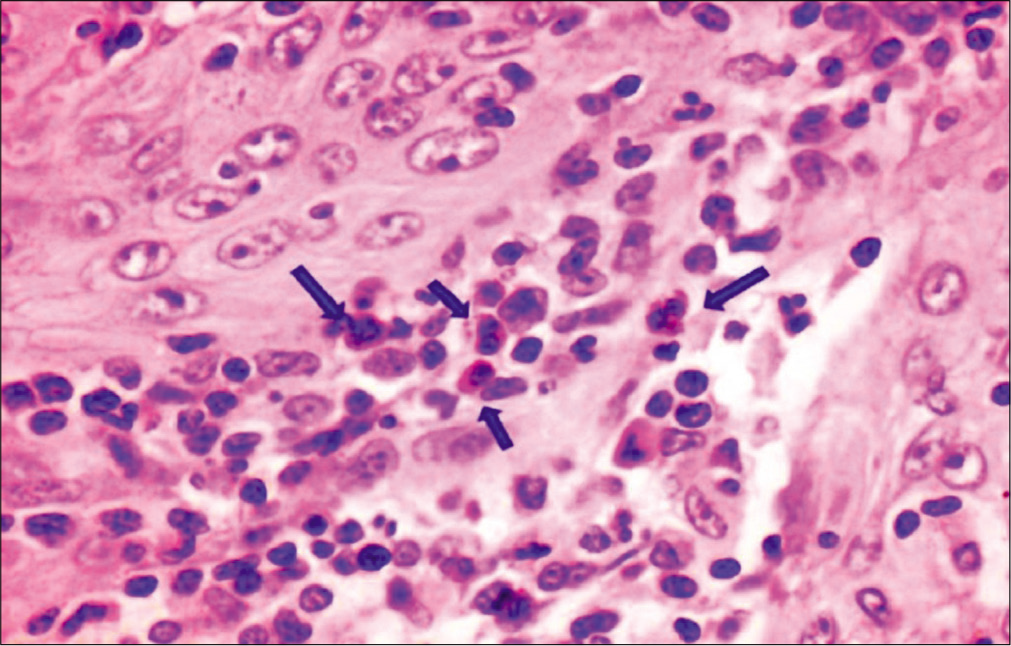
- Tumor-associated tissue eosinophilia in moderately differentiated squamous cell carcinoma (×40), carbol chromotrope stain.
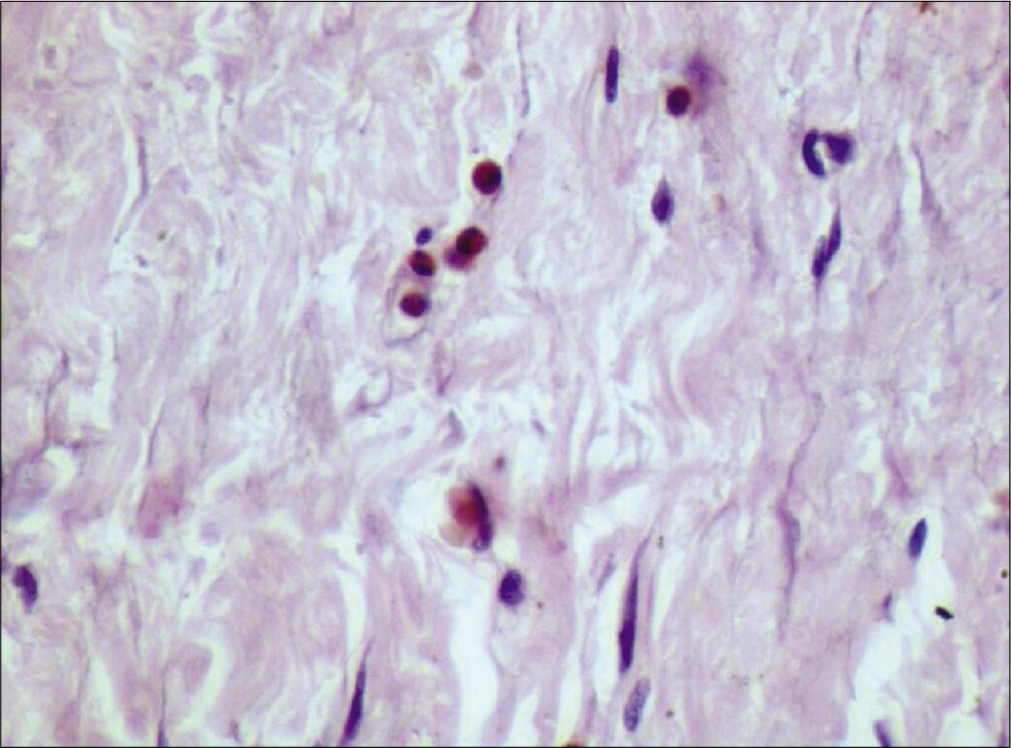
- Tumor-associated tissue eosinophilia in poorly differentiated squamous cell carcinoma (×40), carbol chromotrope stain.
RESULTS
We observed that TATE was present in 87.5% (21 out of 24) cases [Table 1]. We found that there is significant increase in infiltration of tissue eosinophils in OSCC in comparison to normal mucosa [Table 2]. There was no significant difference that was found when the various histologic grades were compared with respective TATE. We observed that TATE is decreasing from well-differentiated OSCCs to poorly differentiated OSCCs.
| S. no. | Normal mucosa | WDSCC | MDSCC | PDSCC |
|---|---|---|---|---|
| 1. | 0 | 83 | 54 | 57 |
| 2. | 5 | 66 | 85 | 16 |
| 3. | 0 | 74 | 124 | 125 |
| 4. | 6 | 20 | 25 | 20 |
| 5. | 2 | 175 | 23 | 28 |
| 6. | 0 | 146 | 62 | 64 |
| 7. | - | 231 | 90 | 68 |
| 8. | - | 63 | 113 | 65 |
| Total | 13 | 858 | 576 | 443 |
| Group | Median | |
|---|---|---|
| Normal mucosa | 1 | |
| OSCC | 233.5 | |
| P: 0.002039 | ||
OSCC: Oral squamous cell carcinoma, TATE: Tumor-associated tissue eosinophilia
The test returned P = 0.002039, which is significant at α = 0.05. This showed a significantly increased TATE in OSCC. From the above data and statistical analysis, we can conclude that TATE count for OSCC is much higher compared to normal mucosa. The median number of eosinophils per 10 hpf in WDSCC, MDSCC, and PDSCC was 78.5, 73.5, and 60.5, respectively. The comparison of TATE in different grades of OSCC did not show much difference between TATE in WDSCC, MDSCC, and PDSCC.
DISCUSSION
OSCC arising from mucosal epithelium of oral cavity accounts for 90% of malignant oral lesions with a 5 year survival rate of 50%. The tumor microenvironment comprises heterogeneous cell populations such as cancer-associated fibroblast, various infiltrating immune cells and subpopulations of non-cell complex communication networks through cytokines, chemokines growth factors, and proteins of the extracellular matrix.[10] Diagnosis at a very early stage still remains crucial as prognosis or survival is directly related to the clinical stage of disease. One of the major drawbacks of clinical staging is its inability to quantify biologic aggressiveness of tumors at a cellular and molecular level. Tumor host interaction is a complex feature of tumor progression including induction of angiogenesis, deregulation of energy metabolism, invasion system activation, evasion of programmed cell death, and suppression of immune mediators. Thus, the tumor microenvironment as an increasingly important target for anticancer strategies is often considered a crucial component in understanding the biologic behavior of a neoplasm.
“Tumor microenvironment” encompasses cellular interactions between cancer cells, immune effectors and inflammatory cells, as well as cells of the tumor vasculature and the stroma.[11] Stromal response usually characterized by the intensity of lymphoplasmacytic infiltration surrounding the tumor plays a decisive role in tumor initiation, progression, and metastasis.[8,12] Increase in the number of eosinophils above 450/cu mm is regarded as eosinophilia. It is often produced as response rather than the actual disease process.[13] Leighton defined TATE as the “tumoral infiltration by eosinophils not related to the presence of necrosis and/or ulceration.”[14]
TATE cells secrete chemical substances such as chemokines (RANTES, endotoxin-1), eosinophil peroxidase, eosinophil-derived neurotoxin (EDN), and cytotoxic proteins such as major basic protein (MBP) and eosinophil chemoattractant protein (ECP) and are capable of activating the immune system through the release of some interleukins (IL) such as IL-2, IL-4, and IL-5. These substances may induce inflammation and cell death and contribute to tumor microenvironment.[15]
It has been postulated that TATE interactions can be due to: (a) Tumor antigenicity-stimulated T lymphocyte; (b) tumor antigens combining with antibodies to form immune complexes; and (c) tumor secretagogues having eosinophil chemotactic ability. Lorena et al. analyzed OSCC with and without TATE and found that eotaxin is a powerful and selective eosinophil chemoattractant. Thus, it was suggested that eotoxin is probably involved in the mechanism of eosinophil chemotaxis to the tumor and in the maintenance of TATE in tumors. In the head-and-neck squamous cell carcinoma, it has been reported that the presence of tissue eosinophils ranges between 22% and 89%. In our study, we observed that TATE was present in 87.5% (21 out of 24) cases, which is in accordance with other studies, they were found intimately associated with tumor cells or with a strong lymphocytic and plasma cell infiltration. We found that there is significant increase in infiltration of tissue eosinophils in OSCC in comparison to normal mucosa [Table 1].
The eosinophils are found to have dual and conflicting function either as promotive or destructive. They can stimulate or inhibit the immune response, leading to a probable good or poor prognosis.[16,17] Various studies have demonstrated their direct tumoricidal activity by cytotoxic proteins and by enhancing the permeability of tumor cells indirectly.[3] Whenever a cell encounters infections or tumor (stimuli) the eosinophils release different substances, such as ECP, MBP, eosinophil peroxidase (EPO), EDN, IL-1, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12, IL-13, IL-18, interferon-γ, tumor necrosis factor (TNF)-α, transforming growth factor (TGF)-α, β, chemokines (RANTES, endotaxin-1), platelet- activating factor, leukotriene C4, neuromediators, and indoleamine 2,3-dioxygenase.[17]
These granulocytes also release pro-angiogenic factors such as basic-fibroblast growth factor, IL-6, IL-8, granulocyte macrophage colony stimulating factor, platelet-derived growth factor, TGF-β, and matrix metalloproteinase-9 on stimulation by TNF-alpha. Therefore, despite having anti-tumor activity, some authors suggested that a more likely possibility is that eosinophils recruited to tumor sites promote angiogenesis and are part of the host connective tissue response to the tissue damage created by the growing tumor.[18,19]
Numerous studies have shown an improved prognosis with TATE in various types of solid tumors, including OSCC, independent of other standard prognostic factors (e.g., stage, age, sex, alcohol or tobacco history, histologic grading, vascularization, vascular invasion, and neural invasion).[20] These studies[4,21-26] favor that increased number of tissue eosinophil associated with anti-tumoral role and shows good prognosis. Furthermore, there have been conflicting reports of TATE as a poor prognostic indicator in OSCC, although it has been suggested that this discrepancy may be related to differences in study methods and design.[27-29] These studies suggested that tissue eosinophils play a tumor promoting role in OSCC. Few studies even suggest that there is no prognostic value of TATE in OSCC.[4,30]
Histopathological grading of tumor is done based on the differentiation of cells as well, moderate and poorly differentiated and is a standard method to assess the behavior of the tumor.[4] In the present study, we observed that TATE is decreasing from well-differentiated OSCCs to poorly differentiated OSCCs, though we did not get the significant difference between TATE in histopathological grades of OSCC. Our findings are in accordance with studies by Alkhabuli and High,[9] where they did not find significant difference between density of eosinophils and SCC differentiation. Similar finding has been recorded by Tadbir et al.[7] and Rahrotaban et al.,[31] they also observed lower TATE count in poorly differentiated group.
Rahrotaban et al.[31] found that TATE was lower in poorly differentiated HNSCC, but correlation between TATE and histopathological grading (Broder’s system) was not statistically significant. This was similar to finding in our study, where TATE count was lower in PDSCC, though difference between TATE counts with MDSCC and WDSCC was not statistically significant. Furthermore, Tadbir et al.[7] did not find any significant relationship between TATE and tumor differentiation in OSCC. The data reported in a study by Simson L et al.[24] provides in vivo and in vitro evidence that eosinophils play an important role in limiting carcinogenesis and/or the growth of chemically induced tumors. Further, they suggest that eosinophils may also play a role in enhancing the methylcholanthrene (MCA) encapsulation process, restricting the contact of surrounding subepithelial tissue with MCA and resulting in a subsequent reduction in cellular mutations, as well as playing an active role in the ongoing immune surveillance process.
In the study by Debta et al., in 2011,[25] they found that in patients who had survived for 3 years or more, tissue eosinophil count is more in comparison to patients who had survived for more than three years. This shows the significant favorable prognostic influence of tissue eosinophil in OSCC with anti-tumoral role, which is in concordance with studies done in by Smith et al.[21,22] and Dorta et al.[23] Jain et al.,[26] in their study, found that mean eosinophil count in non-metastatic OSCC group was found to be significantly higher than metastatic group indicating that eosinophils have a good prognostic role in OSCC.
In contrast, the studies done by Horiuchi et al.,[27] Van Driel et al.,[28] and Wong et al.[29] suggested that tissue eosinophils play a tumor promoting role in OSCC, patients with high eosinophil indices had a statistically significant lower survival than those with lower eosinophil indices. Oliveira et al.[30] found that TATE showed no prognostic value in OSCC and suggested that intense tumor-associated tissue eosinophil seems to reflect the stromal invasion of the OSCCs that occur in advanced clinical stage. Joshi et al.[32] also concluded in their study that there is a strong infiltration of eosinophils in OSCC, but there was no association of elevated tissue eosinophils with overall inflammatory response of the stroma in the specimens studied. A recent study done by Davoine et al.[33] suggest that eosinophils may contribute to the inflammatory response observed in OSCC and limit tumor progression by subsequent anti-tumor activity through the action of cationic proteins. They observed that inhibition of OSCC growth correlated with detectable cytotoxic granule enzyme EPO activity in culture medium.
We observed that TATE is decreasing from well-differentiated OSCCs to poorly differentiated OSCCs. Similar findings were observed in study by Debta et al.[10] High TATE values in WDSCC may be explained as an attempt by the host tissue to resist and limit tumor growth. However, as the tissue resistance was surpassed and the tumor started proliferating, the value of tissue eosinophils decreased as seen in MDSCC and PDSCC.
Similar to our study, Kargar et al.[34] found significant differences in the mean number of eosinophils/mm3 and severity of tissue eosinophila between OSCC and normal groups. Significant difference in the average number of eosinophils/mm3 and severity of tissue eosinophilia between OSCC and normal groups suggests increased presence of these inflammatory cells in OSCC and probably their role in tissue invasion process and progression of OSCC. All these studies suggest that increased infiltration of tissue eosinophils is associated with the favorable prognosis and indicative of an anti-tumoral role of tumor-associated tissue eosinophils.[19]
CONCLUSION
Our study showed a strong correlation of eosinophilic infiltration in OSCC. Evidence of eosinophil infiltration in dysplastic cases should prompt thorough evaluation for invasiveness, especially when evidence of invasion is absent or suspected but biopsy specimens are too small. For proper evaluation of these cells histologically, special stains as cost-effective, rapid, and readily acceptable tools play an important role in diagnostic process. Thus, early identification of recurrence at the time of diagnosis by including the evaluation of tissue eosinophilia as a routine histopathological parameter will be useful in identifying patients at risk and modifying treatment modalities whether TATE as an useful prognostic indicator of oral cancer can only be evaluated by further long-term follow-up of OSCC case studies in the future days. Further improved methods to elucidate the contribution of eosinophils in oral cancer are required since they display a broad range functions.
ACKNOWLEDGMENT
I would like to acknowledge staff members Dr. Vaidehi Narayan Nayak, Dr. Savitha J. K, Dr. Shyamala. K, Dr. Vasumathi. D, and my colleague Dr. Grace Sonia for their continuous support en route for the present study.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Current aspects and future strategies in oral cancer research: A review. J Med Radiol Pathol Surg. 2015;1:8-13.
- [CrossRef] [Google Scholar]
- Role of COX-2/PGE2 mediated inflammation in oral squamous cell carcinoma. Cancers. 2018;10:348.
- [CrossRef] [PubMed] [Google Scholar]
- Tumor associated tissue eosinophilia in oral squamous cell carcinoma: A histo-chemical analysis. Malays J Med Sci. 2015;22:21.
- [Google Scholar]
- Assessment of role of eosinophils in the progression of tongue carcinoma. Asian J Med Sci. 2016;7:17-21.
- [CrossRef] [Google Scholar]
- Tumor associated tissue eosinophilia: A case report with review. Int J Otorhinolaryngol Head Neck Surg. 2017;4:285-8.
- [CrossRef] [Google Scholar]
- Assessment of tumor associated tissue eosinophilia (TATE) in oral squamous cell carcinoma using carbol chromotrope stain. Int J Odontostomat. 2015;9:91-5.
- [CrossRef] [Google Scholar]
- Prognostic significance of stromal eosinophilic infiltration in oral squamous cell carcinoma. J Craniofac Surg. 2009;20:287-9.
- [CrossRef] [PubMed] [Google Scholar]
- Quantification analysis of tissue eosinophilia in squamous cell carcinoma of the head and neck region. Bali Med J. 2018;7:165-9.
- [CrossRef] [Google Scholar]
- Significance of eosinophil counting in tumor associated tissue eosinophilia (TATE) Oral Oncol. 2006;42:849-50.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of myeloid cells (tumor-associated tissue eosinophils and mast cells) infiltration in different grades of oral squamous cell carcinoma. Indian J Med Paediat Oncol. 2016;37:158.
- [CrossRef] [PubMed] [Google Scholar]
- Tumour-associated tissue eosinophilia in oral squamous cell carcinoma-a boon or a bane? J Clin Diagn Res. 2016;10:ZC65.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of infiltration of immunological cells (tumor associated tissue eosinophils and mast cells) in oral squamous cell carcinoma by using special stains. Br J Med Med Res. 2012;2:75-85.
- [CrossRef] [Google Scholar]
- Eosinophils in health and disease: An overview. J Oral Maxillofac Pathol. 2003;7:31.
- [Google Scholar]
- Tumour associated tissue eosinophilia as a prognostic indicator in squamous cell carcinoma of upper aerodigestive tract. Int J Otolaryngol Head Neck Surg. 2016;5:54.
- [CrossRef] [Google Scholar]
- Quantitative assessment of tumor-associated tissue eosinophilia and mast cells in tumor proper and lymph nodes of oral squamous cell carcinoma. J Oral Maxillofac Pathol. 2018;22:145.
- [CrossRef] [PubMed] [Google Scholar]
- Morphometric analysis of the tumor associated tissue eosinophilia in the oral squamous cell carcinoma using different staining techniques. Histol Histopathol. 2003;18:709-14.
- [Google Scholar]
- Eosinophils and oral squamous cell carcinoma: A short review. J Oncol. 2009;2009:310132.
- [CrossRef] [PubMed] [Google Scholar]
- The role of eosinophils and eosinophil cationic protein in oral cancer: A review. Arch Oral Biol. 2011;56:353-8.
- [CrossRef] [PubMed] [Google Scholar]
- Tissue eosinophilia in head and neck squamous neoplasia: An update. Exp Oncol. 2014;36:157-61.
- [CrossRef] [PubMed] [Google Scholar]
- Part II. The prognostic significance of stromal eosinophilia in head and neck cancer. Otolaryngol Head Neck Surg. 1987;96:319-24.
- [CrossRef] [PubMed] [Google Scholar]
- The importance of the eosinophil in head and neck cancer. Otolaryngol Head Neck Surg. 1992;106:27-33.
- [CrossRef] [PubMed] [Google Scholar]
- Tumour-associated tissue eosinophilia as a prognostic factor in oral squamous cell carcinomas. Histopathology. 2002;41:152-7.
- [CrossRef] [PubMed] [Google Scholar]
- Regulation of carcinogenesis by IL-5 and CCL11: A potential role for eosinophils in tumor immune surveillance. J Immunol. 2007;178:4222-9.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of prognostic significance of immunological cells (tissue eosinophil and mast cell) infiltration in oral squamous cell carcinoma. J Cancer Sci Ther. 2011;3:201-4.
- [CrossRef] [Google Scholar]
- Assessment of tissue eosinophilia as a prognosticator in oral epithelial dysplasia and oral squamous cell carcinoma-an image analysis study. Pathol Res Int. 2014;2014:507512.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic factors for well-differentiated squamous cell carcinoma in the oral cavity with emphasis on immunohistochemical evaluation. J Surg Oncol. 1993;53:92-6.
- [CrossRef] [PubMed] [Google Scholar]
- Tumor-associated eosinophilic infiltrate of cervical cancer is indicative for a less effective immune response. Hum Pathol. 1996;27:904-11.
- [CrossRef] [Google Scholar]
- Tissue eosinophilia and its association with tumoral invasion of oral cancer. Int J Surg Pathol. 2009;17:244-9.
- [CrossRef] [PubMed] [Google Scholar]
- P178. Assessment of tissue eosinophilia in head and neck squamous cell carcinoma by luna staining. Oral Oncol. 2011;47:S131.
- [CrossRef] [Google Scholar]
- A histochemical study of tissue eosinophilia in oral squamous cell carcinoma using Congo red staining. Dent Res J. 2013;10:784.
- [Google Scholar]
- Eosinophils in human oral squamous carcinoma; role of prostaglandin D2. J Inflamm. 2013;10:4.
- [CrossRef] [PubMed] [Google Scholar]
- Tissue eosinophilia in oral and cutaneous squamous cell carcinoma and normal oral and cutaneous tissues. J Kerman Univ Med Sci. 2017;24:353-9.
- [Google Scholar]






